A deep look at toxicity turns up less alarm than some of its close chemical relatives. Animal studies point to low acute toxicity, with higher doses producing little more than mild irritation or reversible symptoms. Chronic exposure data remains thin, and that fuels ongoing scrutiny from occupational health monitors. Eco-toxicology finds moderate resistance to breakdown, meaning trace amounts can linger in soils or groundwater if disposal isn’t handled with care. The compound doesn’t show up on lists of known carcinogens or mutagens, but testing continues as more uses spring up in food contact surfaces and biomedical trials. Regular audits and workplace testing keep exposures well below regulatory thresholds, leaning on ventilation, personal protective equipment, and safety signage. Companies that rely heavily on this molecule invest in monitoring programs to spot cumulative environmental loads before they trigger stricter legal action or loss of public trust.
The coming years look busy for anyone working with 2-Ethylimidazole. Growing demand for lightweight, heat-resistant electronics gives it a strong place in supply chains powering everything from smartphones to renewable energy grids. Advanced drug development teams experiment with its core as a launchpad for treatments against resistant infections, encouraged by initial hits in laboratory tests. Circular economy advocates push to redesign both production and disposal, aiming for routes that cut waste or loop byproducts back as raw material. Research groups keep an eye out for any red flags on safety or persistence, eager to update guidelines before regulatory agencies step in. Competition with alternative curing agents nudges chemical producers to sharpen their operations, improve purity, and build up transparent documentation for each batch. The history of 2-Ethylimidazole hints at steady adaptability—each new technical leap builds on decades of steady use and small, deliberate improvements. Scientists and engineers who invest the time into understanding its quirks and advantages find ways to stretch its impact even further, shaping materials and medicines that define how we build and heal in a changing world.
Most people never give a thought to the world of specialty chemicals, but 2-ethylimidazole deserves a closer look. The first time I encountered it, I had spent a summer working at a small electronics assembly shop. My boss, a cautious old engineer, swore by resin systems for all kinds of tasks. If you’ve ever used epoxy to fix a broken object, you might be closer to this compound than you realize.
Epoxy resins make strong bonds, handle moisture, and keep everything solid at high temperatures. Producers lean on 2-ethylimidazole to help bring these resins to life. This little molecule speeds up the curing process. Instead of waiting for hours, manufacturers get tough, finished products much faster. Faster production means less downtime and more output—those are big wins on an assembly line or inside a factory.
So it’s no surprise electronics, aerospace, and construction workers have reasons to care. Printed circuit boards in your phone, for example, owe part of their reliability to special curing agents. With better curing agents, devices keep working longer before they end up in the recycling bin.
2-ethylimidazole also helps researchers and drug developers. Some medicines owe their existence to elaborate steps in chemistry labs—and a handful of those steps need just the right kind of “helper” molecules. I remember one chemist I spoke with at a science career fair saying, “We spend so much energy getting the small details right, and sometimes things hinge on an obscure chemical like 2-ethylimidazole.”
It’s common to see this compound popping up in specialty labs as a building block for more complicated molecules, those that fight infection or inflammation. While not in the final pill, it shapes and guides the chemical reactions that make these medicines possible in the first place.
Not every story about chemicals is rosy. For anyone who has worked in an industrial setting, safety becomes personal. Exposure to imidazoles can irritate skin and eyes. One of my lab partners learned that the hard way, complaining about headaches until the proper protective gear came out. Companies ought to tighten safety standards and researchers must push for greener alternatives wherever possible.
A bigger concern sits with waste and runoff. Specialty chemicals tend to build up in the environment. Waste facilities have to work harder, and calls for biodegradable alternatives keep getting louder. The push for more eco-friendly ingredients isn’t a minor trend—customers and regulators watch for it, and businesses risk falling behind if they ignore these voices.
The world isn’t going to ditch high-performance materials any time soon, but smart choices make a real difference. More businesses test newer, safer curing agents. Chemists rethink formulas to lower risk or improve handling. Open conversations between manufacturers, researchers, and watchdogs keep everyone honest. The payoff isn’t just better products—it’s safer workplaces and a lighter touch on the planet.
So the next time an old cell phone or sturdy plastic gadget crosses your path, spare a thought for all those small chemicals with big behind-the-scenes roles. 2-ethylimidazole reminds us that the simplest compounds can have enormous effects, both good and bad.
There’s something oddly satisfying about the way a small tweak in a molecule’s shape can flip its story. That’s the deal with 2-ethylimidazole. Years ago, when I stumbled upon this compound in a chemicals storeroom, I probably overlooked it for flashier names. A couple of dusty lecture notes later, it was clear this stuff deserves its own attention.
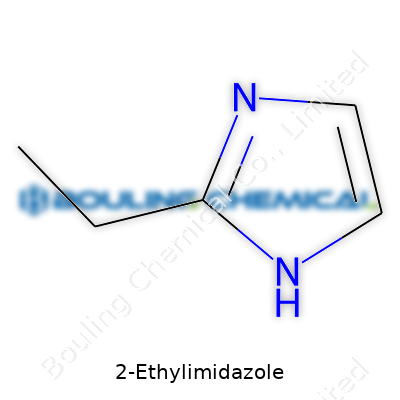
You don’t need to be a chemist to see a puzzle in the name. “Imidazole” tags this molecule as part of a ring family with two nitrogen atoms, bringing to mind caffeine and histamine. The “2-ethyl” part says there’s an extra carbon chain hanging off the second position on this five-membered ring. So instead of a plain, flat molecule, there’s a short carbon tail sticking out, changing how this ring behaves.
Looking at the worksheet of molecular formulas, 2-ethylimidazole comes out as C5H8N2. Here’s how it fits together: There’s a five-membered ring—imagine drawing a pentagon where two corners have nitrogens instead of carbons. Add a two-carbon ethyl group (CH2CH3) to one corner, right at the second position next to a nitrogen atom. This small attachment shifts everything about how the molecule interacts.
People sometimes underestimate little side chains, but from my years running reactions in the lab, those tiny add-ons matter. That ethyl group alters the solubility, shifts the boiling point, and can even tweak how enzymes read the whole molecule. In medicine and industry, those changes can spell the difference between a good reaction and an unusable one.
If you sketch it out, start with the classic imidazole ring: one nitrogen at position 1, a double bond leading to carbon at 2, then another carbon at 3, with a nitrogen at 4 and a carbon at 5. The ethyl group dangles off carbon 2. It sounds simple, but getting the angles right on paper or using modeling kits in class has saved me confusion more than once.
Once you see how small modifications morph a molecule's personality, it's easy to get why chemists keep 2-ethylimidazole in their toolkit. Its mix of hydrophilic and hydrophobic bits plays a big role in chemical synthesis. Pharmaceutical labs rely on these sorts of derivatives when they want to make slight changes without reinventing the wheel. Materials scientists also use these tweaks to control polymer properties, all by swapping or extending functional groups.
Back in school, I found it tough to move from memorizing structures to seeing how shape connects to behavior. Throwing more colored models and story-driven exercises into lessons helped turn vague names into actual mental images. Knowing the formula—C5H8N2—is handy. Still, nothing brings it home like drawing and physically building the ring. Curriculum designers could focus more on that hands-on discovery. For newcomers, online simulators provide an easy way to spin the molecule around and see exactly where that ethyl chunk lands.
Companies speed up research by reaching for compounds like 2-ethylimidazole since small changes unlock so many new combinations. If I could give one tip to students, it’s this: Learn to look past the surface of chemical names. A few added atoms may seem simple, but they lay the groundwork for real-world breakthroughs. The world of chemistry keeps proving that attention to detail pays off, molecule by molecule.
2-Ethylimidazole shows up in a lot of industrial labs, often as a building block for resins or pharmaceuticals. The powder itself isn’t flashy. You might overlook it on the bench next to all the labels in your stockroom. Still, mishandling this chemical can cause problems—both for people and property.
Getting storage wrong with 2-Ethylimidazole can ruin entire batches or, worse, expose staff to unnecessary hazards. At room temperature, this powder doesn’t jump out as dangerous at first glance. Yet moisture can change the story quickly. Keeping the container tightly closed matters—humidity sneaks in fast and clumping makes measurements unreliable.
I always keep these sorts of chemicals in a dry, well-ventilated space. Steel cabinets with clear labels keep things visible, and shelves at eye level mean nobody’s reaching overhead with a wobbly jar. Stick with the original packaging if possible. The manufacturer's containers usually resist both air and light, and repackaging opens the door to mistakes. Folks have tried using unlabeled Ziplocs in the past, but that just leads to confusion and cross-contamination. Invest in proper chemical storage. It saves a world of trouble.
No matter how familiar you get with a chemical, skipping personal protection is a gamble. 2-Ethylimidazole can irritate skin and eyes. Standard practice includes gloves, goggles, and lab coats. In my own routines, I always start with a check: goggles secured, gloves snug, and a fresh coat with fastened buttons. If you end up with dust on your hands, regular handwashing does the trick. Inhaling any fine powder gets tricky—use a dust mask if you’re weighing out larger amounts or if someone jostles the desk.
Showers and eyewash stations close to the work bays become essential too. During a spill or splash, speed matters more than anything, so knowing how to reach these safety features without thinking saves pain and injury. I once had a lab partner misjudge a pour and end up splashing a compound on his wrist. Having clean water nearby made the difference between a minor annoyance and a visit to the nurse. Details like that often separate a safe workspace from a story nobody wants to tell twice.
Good airflow makes cleanup easier and minimizes accidental exposure. In cramped labs, even small spills have a way of lingering in the air. Fume hoods do a lot of the heavy lifting. If the lab lacks that equipment, opening a window and using a fan helps, but isn’t a substitute for proper engineering controls. For me, running procedures under a hood is practically muscle memory by now. Breathing clean air keeps everyone sharp and focused—and sure beats coughing through a shift.
Tossing leftover 2-Ethylimidazole in regular trash just spreads problems outside the lab. Special waste bins, lined and clearly labeled, keep disposal straightforward. Wiping up dust with wet towels stops it from going airborne. Double-checking that nothing’s left behind on the scales or benchtops takes discipline but prevents long-term headaches. Institutions often post clear directions by storage areas. If in doubt, support staff or chemical hygiene officers can walk through the process. No shame in double-checking—you only get one pair of lungs.
Taking care with 2-Ethylimidazole doesn’t come from paranoia—just respect built up over years of handling powders that don’t seem dangerous until the day they are. Open communication and regular check-ins with teammates mean mistakes rarely go uncorrected. If storage or handling rules feel like overkill, ask around for stories and you’ll find someone who can point to the reason for every single step.
Ask anyone tinkering in a lab or working in a specialty chemical plant, and they might nod when you mention 2-ethylimidazole. This chemical builds blocks for curing agents in epoxy resins and shows up in pharmaceuticals too. It’s not famous outside science circles, and most people will never see it up close. Those who do usually wear gloves, goggles, and work in places with fresh air systems humming in the background.
If you spill 2-ethylimidazole on your skin, it burns. Breathing in the powder or dust leaves your nose stinging and your throat scratchy. I know someone who cracked open a drum of this stuff without double-checking his mask—he coughed for hours and spent the night watching for swelling or trouble breathing. People might wonder if it's as drama-filled as sulfuric acid or as infamous as mercury, but the truth is a bit less sensational and no less serious.
According to the Globally Harmonized System (GHS), 2-ethylimidazole carries a health hazard label. Skin and eye contact can spell irritation. In bigger doses or with long-term contact, it causes allergic reactions or inflammation. The data doesn’t point toward it causing cancer, but it hasn’t been studied exhaustively like some other chemicals. Most regulatory agencies, including Europe’s ECHA, flag it for environmental risks too—mostly because aquatic life struggles with this compound if it gets dumped into waterways.
I spent a summer in a chemical plant where simple mistakes, like skipping your dust mask, had consequences. Spend a few hours cleaning up spills without gloves, hands go raw and sore. Some folks took shortcuts, figuring nothing would happen. After a surprise visit from a safety inspector, it turned out that “just dust” meant days off work and mandatory medical checkups. We saw firsthand how easy it is to ignore the warnings, especially when the chemical doesn’t look or smell dangerous.
Most trouble with chemicals starts when people take chances—pouring out powders too quickly, skipping the safety goggles, or leaving empty drums out in the rain where chemicals seep into drains. The easiest fixes prove the most effective. Good ventilation keeps fumes from building up. Gloves and goggles, simple as they seem, turn a hazardous job into a routine one. Training new folks gets overlooked far too often. People learn the hard way, but they shouldn’t have to.
Labeling containers clearly helps: no one wants a mix-up leading to a burn or eye splash. Disposal matters too. Containers don’t get rinsed with tap water and dumped; they follow a chemical waste line straight to regulated sites. A lot of environmental headaches come from careless dumping. Once chemicals reach soil or water, the cleanup lasts for decades. This isn’t just scare talk—it’s happened before, and communities still deal with the fallout.
If workplaces treat 2-ethylimidazole with the respect it deserves, the odds of getting hurt drop sharply. Regulations exist for a reason—people got injured, and governments wrote rules so others wouldn’t face the same fate. It’s easy to focus on big industrial disasters, but small daily mishandlings rack up their own tally of injuries.
Bottom line: 2-ethylimidazole can mess you up if you get careless. With some planning, respect, and the right gear, the risks turn manageable. If society wants safer labs, factories, and waterways, it starts with that simple attitude shift—don’t gamble with chemicals.
If you’ve spent any time in a chemical lab or worked in pharmaceutical manufacturing, you know purity drives a lot of decision-making. For 2-ethylimidazole, this is even more true. Most suppliers offer it above 98% pure, with some batches reaching 99% or better. I’ve handled samples myself where that fraction of a percent made the difference between lab success and wasted effort. Contaminants don’t just disrupt reactions—they can make big flaws slip into your process down the line.
Labs focus so heavily on purity because everything downstream depends on consistency. I’ve seen a project stumble, not because the main ingredient was missing, but because a trace impurity threw off an entire synthesis. For industries like pharmaceuticals or electronics, tight purity keeps the process stable. In my work, I’ve learned to check not just the percentage number on a spec sheet but also which analytical methods a supplier uses. High-Performance Liquid Chromatography (HPLC) or Gas Chromatography (GC) reports always give me extra confidence.
Packaging determines how easy a chemical is to handle, how well it ships, and how much waste accumulates. 2-ethylimidazole arrives most often in sealed bottles, from 100 grams for research up to 25 kg drums for industrial outfits. I remember lugging around those buckets in a plant and cursing every time a bag split open or a bottle cap leaked. So, packaging isn’t an afterthought for me; it means less mess and fewer headaches on-site.
Glass containers do well in the lab since they protect material from humidity and oxygen. I usually pick amber glass if I’m worried about light exposure, although this particular compound holds up fairly well under standard lighting. For larger projects, plastic-lined fiber drums are more common. These won’t shatter if dropped and they’re easier to move around with a pallet jack.
Some suppliers now push for smarter packaging—multi-layer pouches, recyclable plastics, and screw-top barrels with tamper seals. I’ve noticed less spillage and much less worrying about moisture creeping in during storage. One time I saw a pallet that got rained on in shipping; old cardboard boxes would’ve spelled disaster, but the double-sealed drums barely took a scratch. It saved thousands of dollars of inventory right there.
Handling 2-ethylimidazole safely doesn’t just mean slapping a label on a container and calling it a day. Anyone who’s ended up with powder stuck to their gloves or a trace of fumes in the air knows just how important sealed, chemical-resistant packaging is. It makes storing, transporting, and measuring quantities easier, keeping safety standards high and product loss low. Facility managers I’ve worked with pay extra for packing that locks out air, since even small amounts of moisture can clump powders or degrade quality over time.
For those looking at packaging choices, think long term. Spending a bit more up front often translates to less lost product, simpler inventory tracking, and even fewer regulatory headaches. It’s possible to push suppliers for certificates of analysis and detailed packaging info—something I always recommend. A little digging goes a long way in making sure you get what the datasheet promises, without surprises down the road.
Ask reps precise questions: How is the material sealed? What’s the recommended shelf life once opened? Don’t just take volume as the main criterion—consider ergonomics, chemical compatibility, and the conditions it’ll actually face. From my experience, product loss due to shoddy packaging far outweighs the savings of a bargain container. Sometimes splitting large orders into smaller, easy-to-handle packs prevents cross-contamination and keeps material fresher, longer.
| Names | |
| Preferred IUPAC name | 2-ethyl-1H-imidazole |
| Other names |
2-Ethylimidazole 2-Ethyl-1H-imidazole |
| Pronunciation | /tuː ˌɛθ.ɪl.ɪˈmɪd.əˌzɒl/ |
| Identifiers | |
| CAS Number | 1072-62-4 |
| Beilstein Reference | 1209248 |
| ChEBI | CHEBI:85145 |
| ChEMBL | CHEMBL1230531 |
| ChemSpider | 70452 |
| DrugBank | DB04268 |
| ECHA InfoCard | 13e3fae2-4ef4-4ae5-b0d1-c2abdfa0c73d |
| EC Number | 202-506-3 |
| Gmelin Reference | 82519 |
| KEGG | C06534 |
| MeSH | D000068095 |
| PubChem CID | 70038 |
| RTECS number | UJ4375000 |
| UNII | 3J1X7A0U5T |
| UN number | UN2810 |
| Properties | |
| Chemical formula | C5H8N2 |
| Molar mass | 96.13 g/mol |
| Appearance | White to light yellow crystalline powder |
| Odor | amine-like |
| Density | 0.994 g/mL at 25 °C(lit.) |
| Solubility in water | soluble |
| log P | 0.02 |
| Vapor pressure | 0.0062 mmHg (25 °C) |
| Acidity (pKa) | 6.97 |
| Basicity (pKb) | 7.59 |
| Magnetic susceptibility (χ) | -56.5·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.487 |
| Viscosity | 2.10 mPa·s (25 °C) |
| Dipole moment | 1.71 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 179.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -44.1 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3598 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P264, P270, P273, P280, P301+P312, P305+P351+P338, P330, P337+P313, P501 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | 92°C |
| Autoignition temperature | 470°C |
| Explosive limits | Explosive limits: 2.3–19% |
| Lethal dose or concentration | LD₅₀ (oral, rat): 1070 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat 963 mg/kg |
| NIOSH | NIOSH: KV3325000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 200-500 mg/L |
| IDLH (Immediate danger) | IDLH: 100 mg/m³ |
| Related compounds | |
| Related compounds |
Imidazole 1-Methylimidazole 2-Methylimidazole 4-Methylimidazole |