Scientists started tinkering with pyrazine derivatives in the early 20th century, hunting for those rich, roasted, nutty notes that often show up when food gets cooked just right. It didn’t take decades of fussing over bunsen burners for researchers to notice that 2-Ethyl Pyrazine, among all those structural cousins, showed up in a huge range of foods where people craved warmth and comfort, from coffee to cocoa to roasted nuts. Labs and flavor houses soon grabbed it, ramping up synthetic production by the late seventies using basic routes like condensation reactions and figuring out how to boost yields and reduce byproducts. By the eighties, commercial 2-Ethyl Pyrazine rode the wave of snack food expansion, earning spots in chips, instant soups, and countless shelf-stable treats.
Modern manufacturers know 2-Ethyl Pyrazine well. Most batches land as pale yellow liquids with a strong, unmistakable roasted scent. Food companies, aromatherapists, and even tobacco blenders pump it into flavor mixes because it latches onto taste buds, signaling the brain for toastiness, warmth, and richness. The chemical formula for 2-Ethyl Pyrazine, C6H8N2, stays fairly simple, but the impact on product appeal runs much deeper than the sum of those atoms. Few substitutes offer the same instant recognition.
2-Ethyl Pyrazine drips out of the reactor as a clear to slightly yellow oil with a boiling point close to 174–175°C. The molecule smells sweetly nutty and roasted—testers often mention popcorn, cereals, and coffee. It dissolves with some coaxing in water, though not as well as in alcohol or ether. The compound resists breaking down under ordinary storage as long as light and heat stay low. Tinkerers enjoy its robust stability, which lets it survive both shipping and shelf life in a wide mix of conditions. The structure—basically a pyrazine ring with a two-carbon ethyl tail—gives it enough flexibility for further chemical tricks but stubbornness to last through processing.
Regulators demand clear labeling on food-grade batches. Companies submit certificates of analysis showing the minimum purity—typically greater than 98%—to keep off-flavors and unknowns at bay. Documentation tracks batch numbers, CAS number 13925-00-3, and any solvents left from synthesis. Bulk shipments get drums or high-density containers, with tight seals to avoid leaks. In most countries, labels state it as a flavoring or aroma chemical, and safety data sheets travel with each shipment to outline handling, hazards, and first aid notes.
Industrial plants produce 2-Ethyl Pyrazine through reactions starting with ethyl aldehyde and ammonia reacting in the presence of a catalyst. The routes favor liquid-phase synthesis, helping operators control side products that would otherwise stink or turn sour. Most modern operations recycle solvents and dial-in pressure and temperature to squeeze the highest yield per kilogram of reagent. Byproducts get separated in distillation columns or scrubbers, with waste minimized for environmental compliance.
Chemists often use 2-Ethyl Pyrazine as a feedstock to build more complex flavor molecules. Nitration, halogenation, and alkylation give rise to new derivatives, each offering tweaks on the classic roasted profile. Some modifications aim for greater water solubility, while others target stability at high heat—critical for processed foods. Research labs play with cross-coupling reactions to link pyrazine rings together, exploring new scent and taste profiles for niche applications.
Across the globe, suppliers list 2-Ethyl Pyrazine by several names. Some catalogs call it Pyrazine, 2-ethyl-; others sell it as 2-EP or by its chemical number. Food technologists sometimes label it as a roasted flavor enhancer, a popcorn aroma, or a coffee note booster on ingredient lists. Despite the aliases, the chemical fingerprint remains the same, easily traced by any decent quality control lab.
Direct exposure to concentrated 2-Ethyl Pyrazine brings risks. Inhaling vapors or spilling the neat oil on skin can cause irritation or allergic reactions. Workers suit up with gloves, goggles, and well-fitted respirators in poorly ventilated areas. Safety protocols include quick access to eyewash stations and spill kits. Fire hazards stay limited thanks to a relatively high flash point, but manufacturers treat all pyrazines with caution given their strong odors and potential for mischief in the wrong blends. Waste streams pass through treatment plants designed to handle nitrogenous organics, since discharge laws crack down on off-flavors and residues in waterways.
Food and beverage developers reach for 2-Ethyl Pyrazine when formulating anything that needs those instant roasted tones. Snack companies add tiny measured amounts to create the illusion of baked nuts or toasted grains. Coffee roasters use it to round off green or bitter under-roasted batches. Pet food makers blend it into kibbles to mimic the flavor of cooked meats, helping fussy eaters dig in. Beyond food, this compound flavors tobacco blends, fragrances, and specialty teas. Researchers studying aroma in nature also track it as a marker in fire-damaged soils, roasted seeds, and even the breath of some animals.
Flavor scientists run constant experiments to pin down all sensory thresholds for 2-Ethyl Pyrazine, learning just how little is needed to trigger a taste response in different food groups. Some push the boundaries in plant-based meat alternatives, since the molecule brings much-needed depth to otherwise bland proteins. Veterinary researchers study its scent-marking roles, while chemists pursue green synthesis methods that cut waste and energy use. Every year, studies show how the compound mimics Maillard reaction products, giving rise to ever-closer matches for home-cooked comfort foods in industrial kitchens.
Regulatory agencies review animal studies and exposure trials to establish acceptable daily intake levels for 2-Ethyl Pyrazine. Most data points to low acute toxicity at levels used in foods, but chronic exposure at high doses causes concern for liver and nervous system effects. Some studies flag mild cell irritancy, so safety teams monitor cumulative exposures in flavor workers. Regulatory bodies like FDA and EFSA set strict limits, with batch and workplace monitoring routines in place to keep levels well below occupational safety thresholds.
Plant-based foods and low-carbon snacks dominate buyer conversations today, opening doors for new applications of 2-Ethyl Pyrazine. Developers explore its use in lab-grown meats, grain-based seasonings, and sustainable snack coatings. Academic labs seek more eco-friendly production routes, leveraging biocatalysts or fermentation instead of traditional petrochemical origins. As consumer tastes shift toward authentic, comforting flavors—especially as cooking habits evolve—new research and engineering efforts push this compound into broader markets. If science succeeds in lowering production costs and tightens up food safety data, expect to see 2-Ethyl Pyrazine on even more ingredient lists in the years ahead.
If you’ve ever walked into a kitchen and felt that unmistakable scent of roasted coffee or warm, nutty bread, 2-ethyl pyrazine probably played a part. Here’s a compound you don’t hear about often, yet it quietly shapes everyday experiences in food, fragrance, and beyond. Most people don’t realize the hard work a single well-designed aromatic molecule can do.
The food and beverage world leans on 2-ethyl pyrazine because of its roasted, nutty punch. Factories that produce snacks or ready meals sprinkle it in to recreate flavors lost during storage or mass processing. I remember visiting a small flavor lab, where technicians lined up vials of pyrazines to imitate everything from grilled steak to peanuts, letting chefs and manufacturers keep food tasting fresh and appealing even when large-scale production might dull those notes. In chocolate and baked goods, it revives a forgotten depth—those richer, “just-baked” sensations that make a treat so satisfying.
Fast food chains don’t want burgers with bland, reheated meat. Some rely on 2-ethyl pyrazine to lock in consistent, enticing aromas. It saves time, too. Instead of slow-roasting to perfection, manufacturers infuse just the right hints to evoke the same satisfaction of a home-cooked meal. For plant-based products trying to win over meat lovers, this molecule bridges gaps by simulating browned, savory flavors.
Coffee companies searching for the perfect cup often hit a wall during mass production. The distinct notes of a small-batch roast get lost in the shuffle. Here’s where 2-ethyl pyrazine steps in. By boosting roasted and caramel tones, it brings coffee back to life after months on shelves. A cold brew can taste fresh-brewed longer, and instant coffee gains a depth that helps it compete with freshly ground beans. This isn’t just high-end trickery. Companies use science to reintroduce the best parts of real roasting without expensive, waste-heavy processes.
2-ethyl pyrazine isn’t all about food. In fragrances, it mimics the toasted, nutty undertones that make a scent warm and inviting. Imagine a men’s cologne with an edge of roasted coffee or a body lotion carrying hints of hazelnut. These touches create products that stand out in crowded markets where smelling distinct matters. Scent-makers rely on consistency, and this chemical delivers, allowing big brands to keep every bottle or bar smelling the same, even if raw natural extracts change with harvests and weather.
Every time I hear about another synthetic flavor, questions come up about safety. The FDA puts 2-ethyl pyrazine on its list of approved flavoring agents. Studies haven’t flagged worrying health risks at levels used in foods. Still, some consumers worry about anything artificial. Producers could answer these concerns with clearer labeling and transparent sourcing. The push for “natural” options nudges researchers to explore ways to make pyrazines through fermentation rather than petrochemicals, shifting toward cleaner production methods for both health and environment’s sake.
More industries want to craft unforgettable flavor and aroma experiences—snacks, beverages, personal care. 2-ethyl pyrazine keeps finding new applications. Whether inside a chocolate chip cookie or in a bottle of aftershave, it shapes what we taste and smell more than many people suspect. The smartest use balances flavor, safety, and sustainability, so every cup of coffee or bite of bread delivers on the promise of satisfaction.
There’s something fascinating about tracing food flavors back to individual chemicals. 2-Ethyl pyrazine shows up in all sorts of places—from roasted nuts to the warm edge of cocoa or coffee. If you look at its chemical roots, the formula comes out as C6H8N2. The numbers are pretty simple, but behind them sits a world of sensory experience. As for weight, its molecular mass runs close to 108.14 g/mol. Every time this compound pops up in an ingredient list, it’s not there in huge quantities; a pinch changes everything.
Most people don’t realize how much science goes into the taste of that piece of toasted bread. When grains hit high heat, a lot can happen. Pyrazines, especially the ethyl kind, give food a stronger, nuttier personality. Imagine biting into toasted cashews—there’s a rich depth that isn’t just about salt or oil. After watching chefs roast seeds or nuts, I’ve seen a shift in color and noticed the aroma lifting from the pan, signaling that these tiny molecules are getting to work.
In processed foods, the addition of 2-ethyl pyrazine can give a quick flavor punch without months of aging or slow roasting. I remember reading about snack makers using it to mimic slow-roasted flavors—making even budget chips or crackers carry some of that real-roast energy. Its low threshold means even a tiny amount does the trick. Still, as much as folks in food labs like to tinker with flavor, there’s always pushback about what “natural” should mean.
No one wants to eat something with a five-syllable name they’ve never encountered in nature. I’ve run into people turning a suspicious eye toward any additive once they spot numbers and letters rather than a word like “salt” or “cocoa.” The reality for 2-ethyl pyrazine is pretty reassuring. The Joint FAO/WHO Expert Committee on Food Additives checked it for toxicity and gave it a thumbs-up in the usual food-use amounts. It sticks around in such small doses that no red flags have shown up—even in long-term studies, effects remain absent.
Still, transparency counts for a lot. Listing what’s in food and spelling out its source—whether it’s coming from a lab or a roasted bean—helps break down suspicion. Years ago, as labeling laws shifted, the industry felt some heat about being too vague, and the clearer disclosure helped many shoppers trust what they buy again. Even though 2-ethyl pyrazine is safe, letting people know why it’s there, in plain language, builds confidence.
Plenty of cooks and home bakers chase authentic taste, working toasting and browning into recipes instead of relying on additives. Learning about compounds like 2-ethyl pyrazine made me pay attention to how much flavor really depends on the way you process food. Using heat to generate complex molecules isn’t a new trick—it’s how civilizations figured out grilling and searing long before chemists mapped out the structures.
There’s value in knowing what’s inside the food package, not just for safety but for curiosity. The formula—C6H8N2—might never show up on Grandma’s recipe card, but it plays a quiet part in making dinner smell and taste right. For anyone curious about why roasted nuts taste different from raw ones, a trip into molecular science gives answers regular ingredient lists can’t.
Walk through the ingredient list on your snack bag and you might spot 2-Ethyl Pyrazine. This compound shows up in roasted flavors, chocolate, coffee, nuts, and even some savory sauces. The reason chemists and food technologists like it revolves around its punchy, nutty, almost warm taste profile. Ever smelled roasted peanuts or fresh-baked bread? Those rich, toasty notes often point back to molecules like this one.
The big question always comes down to safety. Most people trust their food, and that trust rests on organizations like the U.S. Food and Drug Administration (FDA) and the Joint FAO/WHO Expert Committee on Food Additives (JECFA). Both groups have reviewed 2-Ethyl Pyrazine for decades. Based on current research, they label it as safe within the usual amounts that end up in food.
From a toxicology angle, researchers have poked and prodded this flavor ingredient in lab studies. High doses beyond what you’d ever get in your average snack have not led to mutations or toxic effects in rats and mice. Scientists have reason to keep watch, but these tests back up a record of safe use. Folks working in food sciences are sharp enough to check for problems, since no one wants lawsuits or sick consumers.
Having watched trends in food science, it’s clear rules and regulations set a floor but not always a ceiling for safety. Just because the FDA signs off on something today doesn’t mean new data won’t shake things up tomorrow. Think about other ingredients once considered no problem—over years, evidence can pile up and shift opinions. That’s not to inspire panic, but a bit of healthy skepticism helps.
One practical way people can protect themselves is by reading ingredient lists and pushing for transparency. No one wants to eat a science experiment without consent. When companies label things clearly and keep serving sizes reasonable, that builds back trust.
2-Ethyl Pyrazine isn’t out to get anyone. Used in tiny amounts, it helps foods smell and taste more delicious. Problems pop up with dosage or if people develop sensitivities. The bigger trouble for most eats comes from overeating processed snacks, not from the flavor molecule itself. That’s something most home cooks notice: focus on whole foods, toss in flavorings sparingly, and meals tend to land better for health.
Public demand actually shapes policy and product choices. I’ve watched brands ditch artificial flavors altogether or switch to more natural sources just because buyers started asking hard questions. No one single molecule defines the safety of our diet, but every voice steering toward real, honest ingredients pushes the industry in the right direction.
Checking the science along with personal gut instinct, most people have good reason to see 2-Ethyl Pyrazine as safe. Ongoing study is key. Scientists need to keep poking at theories, and folks at home should keep speaking up about what they want in their food. Supporting more research and calling for even clearer food labels makes sense. Everyone deserves to know what’s in their snacks, right down to the last roasted note.
Anyone who’s spent time in a lab notices how some compounds demand attention every step of the way. 2-Ethyl Pyrazine belongs in that group. I remember working with food flavorings and fragrances where this chemical added that nutty, roasted aroma everyone loves in chocolate or coffee. That pleasant smell masks how touchy these chemicals can be once the bottle is open.
A little bit of carelessness with storage can turn an expensive ingredient into a problem for both people and products. Vapors drift, containers corrode, and odd things happen if the basics get ignored. You don’t want to walk into your storeroom and find that signature smell isn’t so pleasant when it’s everywhere.
Dry air and a steady, cool temperature serve as a chemical’s best friends, especially for organic compounds like 2-Ethyl Pyrazine. Humidity leads to clumping, leaks, or even nasty reactions. I’ve seen glass bottles coated with droplets after someone left a cap loose near a steam vent — a mess nobody wants to clean.
Chemical supply rooms usually set thermostats between 15-25°C. That’s not just for the comfort of workers. It helps slow down the tiny reactions that start when chemicals get too hot or cold. Sticking close to this range means fewer surprises with evaporation or container breakdown.
UV and sunlight break down a lot of fragrant chemicals long before expiration. Years back, I tried to save space by putting some flavor compounds near a window. Not only did the fresh batch lose scent, but the liquid inside took on a strange hue and stank up the whole workbench. Block out sunlight with cabinets or opaque containers. Your nose and budget will thank you.
Nobody likes opening sealed bottles or fighting sticky lids. With 2-Ethyl Pyrazine, you can’t ignore this detail. The chemical evaporates more easily than plain water, which means leaky containers lead to strong odors fast. Choose glass or high-density polyethylene bottles with reliable seals. I’ve seen rubber stoppers soften and warp when left for months — stick with screw-on or clamp-style tops for best results.
Even with perfect storage, spills and slow leaks happen. I’ve had to air out spaces where someone stored pyrazines without enough ventilation. That nutty smell starts to burn your nose quickly. Good airflow from fans or ventilated storage cabinets keeps working conditions safe and stops vapors from traveling further than intended. Modern chemical storage rooms often use vent hoods or extractor fans for this reason — not fancy extras, but real necessities.
I once grabbed the wrong bottle thinking it was a similar-smelling chemical. No real harm done, but it underlined how easy things can go wrong. Clear, bold labels make life simpler, especially under pressure. Store 2-Ethyl Pyrazine away from acids, bases, or oxidizers to dodge accidental reactions. Keeping each family of compounds separate might use more shelf space, but it avoids headaches down the line.
Safe handling always depends on more than a locked door. Quick reminders go a long way: run training, walk through storage rooms, review spill clean-up steps regularly. Experienced workers spot unsafe setups faster than any sign on the wall. Mixing real stories into safety meetings — cases where rules prevented expensive or dangerous mistakes — helps new team members buy into good habits from day one.
All told, 2-Ethyl Pyrazine behaves well if you show respect for the details. Consistent care with storage protects not just the chemical’s quality, but also every person sharing that workspace.
No one is calling 2-Ethyl Pyrazine flashy. This compound quietly shows up in labs and, for most people, stays invisible in daily life. It looks like a pale yellow liquid or sometimes appears almost colorless. Anyone expecting a violet shimmer or something dramatic would be disappointed. The simplicity tells a bigger story, though. Chemists trust consistency, so a familiar, clear appearance reduces guesswork. There’s comfort in knowing what you’ll see—essential in a world where surprises can ruin a day’s work.
Stick your nose anywhere near a bottle of 2-Ethyl Pyrazine and you’ll find the real personality. This chemical carries a powerful, nutty, roasted aroma that slips right into your senses. It’s the same scent that hangs around fresh roasted coffee or toasted grains. In my years of working with flavor labs, I've watched folks nod in recognition the moment they crack open a vial—the smell jumps out, grabs your attention, and lingers in the air. This aromatic intensity doesn't just help during product design; it also aids in safety. If there’s a leak, the human nose picks it up almost instantly. You can't ignore the odor, making it one of the safest kinds of warnings that chemistry offers.
Pour 2-Ethyl Pyrazine into water, and you’ll see an unfriendly response. This compound doesn’t want to dissolve much at all in water. Its molecules prefer to stay with their own kind, separating themselves from water droplets. Toss some into oil or alcohol, and the story changes—here, it blends in easily. Anyone formulating food flavors or fragrances knows the headaches this brings. You can’t just dump it into soda or a water-based solution and expect even results. I once watched a team fight for hours trying to disperse the stuff into a drink mix. The challenge inspires creative workarounds—sometimes, people build fancy emulsions or use alcohol as a carrier. Problems spark innovation, and the solubility quirks of this chemical push folks to experiment with different delivery systems.
Physical traits aren’t just interesting facts—they control everything from how a chemical gets stored to where it’s useful. For 2-Ethyl Pyrazine, the roasted, nut-like odor finds a home in food science. Cereal manufacturers chase that freshly-baked aroma. Snack makers tap into those same notes to mimic toasted nuts. Rapid odor recognition also matters beyond flavor. If you’ve got a spill, strong scent helps teams act fast before minor issues grow into health risks.
Solubility, or the stubborn lack of it in water, keeps things interesting. This property shakes up planning for food or industrial use. Companies invest real time and money into building solutions that coax this compound into places it normally refuses to go. Scientists have whipped up new emulsifiers or blended alcohol-based carriers that safely tuck away strong odors until just the right moment. Every oddball property pushes the field ahead—sometimes one sticky compound changes the way a whole category of products is made. That’s the real reason why learning about these physical quirks sparks so much attention.
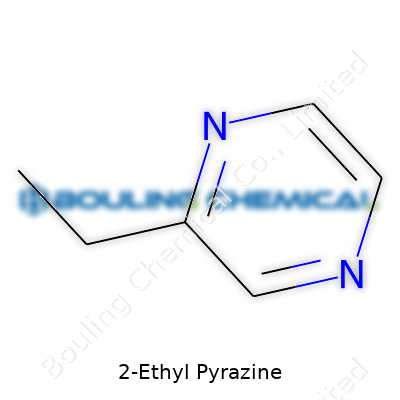
| Names | |
| Preferred IUPAC name | 3-Ethylpyrazine |
| Other names |
2-Ethylpyrazine Pyrazine, 2-ethyl- 1-Ethylpyrazine |
| Pronunciation | /tuː ˈɛθɪl paɪˈræziːn/ |
| Identifiers | |
| CAS Number | 13925-00-3 |
| 3D model (JSmol) | ``` 3Dmol.js?model=modelData&format=mol&modelData=JSmol_pdb_string_data ``` |
| Beilstein Reference | 361873 |
| ChEBI | CHEBI:34570 |
| ChEMBL | CHEMBL187547 |
| ChemSpider | 57311 |
| DrugBank | DB14083 |
| ECHA InfoCard | ECHA InfoCard: 100.018.805 |
| EC Number | 202-591-2 |
| Gmelin Reference | 61787 |
| KEGG | C06727 |
| MeSH | D010527 |
| PubChem CID | 12250 |
| RTECS number | UJ4375000 |
| UNII | 5A6N8WQ224 |
| UN number | UN2810 |
| Properties | |
| Chemical formula | C6H8N2 |
| Molar mass | 122.16 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | nutty, popcorn, roasted |
| Density | 0.982 g/cm³ |
| Solubility in water | Soluble |
| log P | 0.67 |
| Vapor pressure | 0.13 mmHg (at 25°C) |
| Acidity (pKa) | 4.60 |
| Basicity (pKb) | 2.70 |
| Magnetic susceptibility (χ) | -49.6×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.5070 |
| Viscosity | 1.100 mPa·s (25 °C) |
| Dipole moment | 1.93 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 318.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -22.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3775.9 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 62°C |
| Autoignition temperature | 540 °C |
| Explosive limits | Explosive limits: 1.8–12% |
| Lethal dose or concentration | LD50 (oral, rat): 740 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 1,430 mg/kg |
| NIOSH | TT2975000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 5 mg/m³ |
| IDLH (Immediate danger) | Unknown |
| Related compounds | |
| Related compounds |
1-Ethylpyrazine 2,3-Dimethylpyrazine 2-Methylpyrazine Pyrazine |