Tracing the roots of 2-Ethyl-6-Methylpyrazine takes us back to flavor chemistry’s early days, when food scientists started isolating and naming volatile compounds responsible for roasted, nutty, and earthy notes in processed foods. The world got a real understanding of the chemistry behind scent and flavor as pyrazines came onto the radar in the mid-20th century. Researchers digging through samples from roasted coffee, baked bread, and cocoa found a pattern. The distinct aroma, the signature profile, led them to this pyrazine variant, and pretty soon labs started producing it synthetically. Once mostly theoretical, these discoveries began forging stronger links between aroma compounds and their precise structures, helping industries improve aromas and flavors for mass markets.
2-Ethyl-6-Methylpyrazine earns its reputation in the food, beverage, and fragrance industries for the punch of roasted, nutty, and earthy scents it brings to the table. This molecule gives an unmistakable top note that coffee connoisseurs and chocolate makers aim for. It also tends to show up in tobacco flavoring and as a marker in environmental studies for fire or roasting processes. You typically find it in colorless to pale yellow liquid form, shipped in tightly sealed dark glass or metal containers to keep air and light out—the trick is keeping the purity and aroma intact during storage and transport.
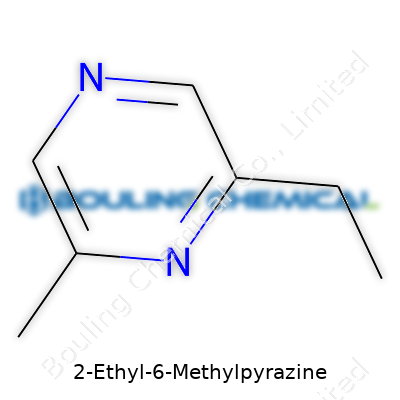
Inside the lab, this compound takes the form of an oily liquid, shining under the light and carrying a boiling point usually around 170-180°C. Its molecular formula, C7H10N2, translates to a structure that balances volatility and stability, making it easy to blend with other flavor additives. 2-Ethyl-6-Methylpyrazine doesn’t dissolve well in water but goes gladly into most organic solvents, from ethanol to diethyl ether, which lets formulation chemists choose solvents depending on application needs. It gives off a strong odor even at low concentrations—the classic pyrazine punch prompts both praise from flavorists and concern from safety regulators looking at exposure thresholds.
Every bottle or drum carries labels outlining purity (usually above 98%), batch number, storage advice, and handling symbols. Manufacturers almost always offer a detailed spec sheet, which includes limits on residual solvents, heavy metal content, and moisture levels. International standards, such as those from the FCC or JECFA, support labeling accuracy, connecting food safety guidelines directly to these spec sheets. These aren’t bureaucratic hurdles—these details safeguard production lines against contaminants and keep supply chains accountable. The focus stays on clear chemical identity, shelf-life, and compatibility information to support QC checks all the way from lab buying to consumer product.
Most industrial production methods rely on catalytic cyclization reactions of appropriate alpha-diketones and amines, fine-tuned through decades of process chemistry. Instead of relying solely on extraction from complex matrices like coffee or cocoa, big producers set up reactors for controlled synthesis. Feedstocks like ethyl- and methyl-substituted amines meet glyoxal or similar compunds under inert atmospheres, with specific catalysts steering the process to favor the 2-ethyl, 6-methyl isomer. The technical know-how focuses on getting high yield and purity while driving down side products, and this process design stays at the heart of supply chain stability for flavor manufacturers around the world.
Chemistry minds look at 2-Ethyl-6-Methylpyrazine not just as an end product but as a starting point for creating tailored molecules. You see labs functionalizing its pyrazine ring, swapping out the ethyl or methyl group for longer or branched chains, or adding oxygen-containing groups for higher water solubility. The chemical backbone withstands mild oxidation and reduction, letting manufacturers tweak the aroma character or solubility for a wider range of foods and fragrances. There’s an ongoing search for biocatalytic or green chemistry approaches—using enzymes or benign solvents—reflecting the growing demand for cleaner production techniques, even as traditional methods still dominate the space.
Anyone sifting through ingredient lists may run into names like 2-Ethyl-6-Methylpyrazine, 6-Methyl-2-ethylpyrazine, or simply EM-Pyrazine. Some flavor compendia label it under proprietary names tied to major suppliers, reflecting subtle formulation tweaks. This array of synonyms often confuses non-chemists, but cross-referencing CAS number 13360-64-0 untangles the mess. An industry that runs on trust needs this level of transparency—even with clever branding strategies popping up in technical data sheets, the core structure keeps its identity wherever you look.
Handling strong-smelling, volatile chemicals in a plant or lab isn’t something to take lightly. Regulatory agencies have drawn up workplace exposure guidelines, and these get reinforced by in-house SOPs like: wear gloves, keep the room ventilated, and watch out for accidental spills. Safety data sheets point out the moderate irritancy of the pure liquid and the risks posed by long-term inhalation above threshold levels. I’ve seen specialty producers install local exhaust systems just for working with powerful aroma compounds—there’s no hiding from quality control or worker safety. In finished consumer goods, residual levels undergo strict testing to guard even sensitive consumers.
The allure of 2-Ethyl-6-Methylpyrazine runs primarily through food and beverage manufacturing. From coffee, chocolate, and nut flavors to roasted meat marinades and bakery glazes, this molecule serves as a cornerstone for recreating or boosting natural aromas. The tobacco industry values it for delivering the backbone of toasted and caramel-like notes. Every time a food technologist aims for the comfort of oven-fresh baked bread in a packaged product or the complexity of roasted nuts in a dairy-based treat, EM-Pyrazine comes into play. Perfume houses and home fragrance brands also borrow from its aroma, using it to add warmth and depth to new compositions. Environmental scientists trace its presence in fire-impacted areas, using its fingerprint to understand ecosystem shifts after wildfires and industrial emissions.
Ongoing R&D focuses on cleaner production pathways, lower-impact solvents, and safer, renewable raw materials. Researchers in both academia and commercial labs keep pushing for milder reaction conditions, aiming for processes that minimize waste and side products. There’s also a real move towards biotechnological approaches, expressing enzymes that mimic nature’s own routes—mining bacteria or fungi for biocatalysts that craft the pyrazine skeleton from simple sugars or amino acids. Every advance here filters down the supply chain, shaping the sustainability footprint of global flavors.
The question of safety goes deeper than regulatory paperwork. Toxicologists run repeated-dose studies, in vitro mutagenicity assays, and food allergenicity screens to put hard numbers behind allowable dose limits. Reports show that flavoring levels in finished goods stay well below any thresholds that cause concern for acute or chronic effects. At the same time, safety researchers don’t let up—reference doses get revisited any time someone uncovers new animal or human data. A responsible industry must handle trace contaminants that pop up in synthetic routes, especially heterocyclic amines. Even as standards tighten, the core safety picture supports continued, careful use in flavor work.
Looking ahead, the industry gears up for tighter regulation, evolving consumer scrutiny, and pressure to source ‘natural’ flavor molecules. Efforts to engineer yeast, bacteria, or fungi to produce these molecules in fermentation tanks gather momentum. I see a shift from traditional chemical manufacturing towards new systems biology approaches for tuning flavor profiles straight from renewable feedstocks. At the interface of food tech, green chemistry, and health science, companies race to maintain product performance while shrinking the environmental footprint. All the while, consumer demand for authentic, robust taste in both plant-based and classic foods keeps demand for 2-Ethyl-6-Methylpyrazine alive and well.
Most people never realize how many ingredients shape their meals before anything hits a pan. One tiny but mighty organic compound, 2-Ethyl-6-Methylpyrazine, usually goes unnoticed by everyday cooks and eaters, yet it helps create food memories all the time.
This molecule shows up in flavor labs around the globe. It forms one of those crucial pieces in the puzzle that is roasted, grilled, or baked goodness. Its structure belongs to the pyrazine family, which chemists and chefs alike connect with deep, nutty, and earthy aromas. Ever noticed how popcorn just explodes with that warm, toasty scent? Pyrazines often play a big part.
Food companies add 2-Ethyl-6-Methylpyrazine to recipes when they want more than the basic taste from natural ingredients. Roasted nuts, baked cookies, barbecue chips, and coffee all seem richer thanks to aroma boosters like this one. It packs a punch in tiny amounts—for folks working in food development, it’s a familiar part of making something ordinary taste freshly toasted or perfectly browned even after months on a shelf.
That flavor punch comes from some serious science. These pyrazines form naturally when heat transforms sugars and proteins during cooking—a process known as the Maillard reaction. But large-scale food producers can’t always rely on heat and time. They need consistency, so they reach for pure flavor molecules like this one. In my own kitchen, chasing that just-roasted taste in slow-cooked dishes rarely matches the efficiency of adding an expertly crafted drop of concentrated flavor.
2-Ethyl-6-Methylpyrazine doesn’t stop at potato chips and cereals. The fragrance industry knows it for its nutty, woody, and earthy notes. It turns up in some perfumes and personal care items, giving a grounded, almost homey scent. One might not expect to find anything reminiscent of baked bread in a bottle of cologne, but pyrazines keep surprising.
Even non-food uses deserve attention. Rodent control formulas sometimes use pyrazines to mimic danger signals—these scents warn mice and rats away. Wildlife researchers have drawn on this to manage animal behavior without using poison.
People may worry about lab-made flavor chemicals, picturing unpronounceable names and strange side effects. Regulatory agencies like the FDA and EFSA have cleared 2-Ethyl-6-Methylpyrazine for use in foods at specific, tiny levels. Current evidence points away from health risks when eaten in amounts people actually encounter. Still, broader questions about ultra-processed foods linger. Heavy reliance on additives, even safe ones, has fueled debates on nutrition and transparency.
Sourcing and production methods raise their own issues. Some pyrazines come from petrochemical feedstocks. Pushing for greener, bio-based routes could help lighten the footprint, while closer track on labeling keeps eaters in the loop. My own shopping habits shifted after learning about hidden ingredients, so clear and honest packaging really matters over time.
2-Ethyl-6-Methylpyrazine offers a window into the hidden architecture of flavor and fragrance. Unlike the starring ingredients you pick up at the farmers’ market, it works backstage, making packaged food more appetizing or perfumes more memorable. Small molecules like this shape taste and scent in ways we don’t always expect. As food culture moves forward, putting these behind-the-scenes players in the spotlight could help everyone pick and enjoy meals and products they feel good about.
2-Ethyl-6-Methylpyrazine sounds like something for a lab, but its aroma and flavor bring me right to the dinner table. Open up a jar of roasted nuts and breathe in deeply—that dry, earthy, roasted note isn’t just magical culinary intuition. This compound is a key player behind that rich, familiar scent.
I grew up in a household where toasting bread before breakfast was ritual, and that moment when the crust turned golden always promised comfort. Pyrazines as a group help create that roasted aura. 2-Ethyl-6-Methylpyrazine stands out for its nutty, earthy, slightly green character. Stick your nose near a pan of roasted peanuts or a handful of cocoa nibs. That warm, baked bread, almost caramelized, yet not sweet, finds representation here.
Biting into a well-toasted slice of wheat bread, you taste more than just wheat—there’s a depth to the crust, something full-bodied with hints of potato skin and even a bit of bell pepper. Grilled steak, roasted coffee beans, dark chocolate—all of them owe part of their signature profiles to molecules like 2-Ethyl-6-Methylpyrazine. That same molecule turns up in cooked vegetables, lending a savory backbone. It never reads as flashy—no sugary top notes, nothing that drowns out other flavors. Instead, it brings harmony, a mellow and grounding warmth.
One afternoon working in a coffee shop, the aroma during a batch of dark roast always drew a small crowd. People would wander in, noses lifted, pulled by scents evocative of campfires and early mornings. It isn’t nostalgia or marketing—compounds like this do heavy lifting, shaping what we think of as “deep roast” flavor. Our senses of smell and taste lean on these pyrazines to signal comfort, fullness, and satisfaction.
Food companies and chefs study these molecules for a reason. Develop a snack with that signature “freshly roasted” aroma, and people reach for it again and again. Artificial flavors had a bad reputation for years, but learning to use molecules already found in nature—like this one—lets products stay true to their roots. Instead of trying to mimic “something good,” they’re building on what’s already real.
Is the flavor profile always “roasted”? Not quite. 2-Ethyl-6-Methylpyrazine brings a sort of green bell pepper note at higher concentrations. Think about walking through a greenhouse warmed by the sun, that slight earthiness blending with greenery. At lower doses, you probably just taste the nuttiness, the edge of toast, or the dark core of a coffee bean.
If companies start overusing the molecule, foods can tip into bitterness or an unnatural, overly-processed taste. The challenge is restraint and balance. Cooks and product developers who respect this find the sweet spot—enhancing natural flavors, not glossing over them.
In my kitchen, toasting nuts a bit too long gives a whiff of harshness, almost singed—proof that more isn’t always better, even with star molecules like this. The lesson is simple: let natural flavors shine, and only use what lifts them up. Consumers today notice authenticity, and success comes from getting that balance right. The aroma profile of 2-Ethyl-6-Methylpyrazine proves that real depth comes from subtlety and restraint, not just volume.
Open up a bag of chips or a box of cookies, and chances are you’ll find an ingredient list that seems more at home in a chemistry lab than a kitchen. Among those big names, 2-ethyl-6-methylpyrazine pops up from time to time, especially in flavored snacks, roasted nuts, and processed cheese. This chemical sounds intimidating, but its job is pretty simple: it brings a roasted, nutty, or earthy note to food. Some folks call it a “flavor enhancer.” Food scientists appreciate how it can boost that fresh-from-the-oven aroma in mass-produced foods.
Food safety decisions fall to groups that dig into toxicology and exposure. In the US, the FDA keeps tabs on everything in our food—even additives with tongue-twisting names. The FDA considers 2-ethyl-6-methylpyrazine “Generally Recognized as Safe,” or GRAS, for use in food at typical amounts. Similar bodies in Europe, like EFSA, review the same data and reach comparable conclusions. I’ve read through some of their reports, and the science crowd usually agrees: at really low concentrations—think parts per million—this ingredient isn’t likely to cause harm.
Still, the comfort of seeing “approved” or “safe” on paper doesn’t always make people feel at ease. There’s history behind that cautious attitude, and it’s hard to blame consumers for worrying. After all, many people think food should sound familiar, not like it needs a glossary.
Here’s where the tests kick in. Researchers look for signs of toxicity, cancer risk, and allergic reactions. In studies where lab animals got huge amounts of 2-ethyl-6-methylpyrazine—much more than you’d ever find in the real world—no alarming effects showed up. At the tiny levels used for flavor, scientists just aren’t seeing anything dangerous. Food allergy groups haven’t raised red flags, either. Still, the research isn’t endless, and new data sometimes reshape old opinions.
I remember a time I worried about any “synthetic” flavor, thinking “If I can’t pronounce it, I probably shouldn’t eat it.” That sort of fear isn’t unusual, especially now, with social media amplifying every food scare and trend. But reading the raw evidence helped me calm down a bit. A lot of these chemicals exist in cooked food naturally—pyrazines sometimes form in your kitchen, just from roasting nuts or baking bread.
The debate isn’t just about one ingredient. The bigger question hangs over all processed food: do we trust what’s added to our meals? Some folks see every new chemical as suspect, while others trust science and regulation to keep foods safe. Somewhere in between lies a balance point.
People with a taste for whole, simple food will always prefer snacks with no wild-sounding ingredients. Maybe they turn to baking at home, or hunt for “clean label” products. On the flipside, plenty of families rely on processed food for cost, convenience, and shelf life. Companies add flavors like 2-ethyl-6-methylpyrazine to improve taste and keep those foods appealing.
Trust in food hinges on transparency. Clear labels help; so does sharing research, not just repeating blanket statements like “Safe at approved levels.” Open access to independent studies goes a long way. If food makers offered up plain-language info on these ingredients, more people might relax. For those with lingering doubts, supporting more ongoing research keeps everyone honest and makes future decisions better informed.
In my own kitchen, I try to mix it up: fresh ingredients most days, but no panic if I snag a pack of flavored almonds. Knowing the science, and looking for progress on transparency, helps me sort out what matters most at the table.
Nobody forgets the first time they open a tiny bottle and meet the roasted scent of 2-Ethyl-6-Methylpyrazine up close. One drop can sweep through a whole kitchen, delivering roasted, nutty, almost burnt-sugar notes. At first, it feels like overkill—how does anyone measure such a strong aroma? But dialing in the dose starts to look less mysterious after a few rounds with the pipette and a handful of test batches.
Walk into any R&D food lab, and you'll spot pyrazines being handled with care. For 2-Ethyl-6-Methylpyrazine, most food technologists keep to a typical range of about 0.01 to 2 parts per million (ppm) in finished products. Roasted nuts, coffee, and cooked meat flavors benefit from lower levels—often close to 0.1 ppm or even less. Snacks that need extra character, like seasoned chips or nut-based spreads, might reach as high as 1.0 ppm. That higher end isn’t common, though; beyond that, the flavor gets overwhelming, and bitter metallic notes creep in.
Beverages follow a tighter rulebook. Even 0.05 ppm can transform a bland malt drink. The same holds for confectionery: chocolate and caramel flavors depend on using small amounts—sometimes below the threshold of 0.05 ppm—otherwise the result doesn’t taste like a treat anymore. Perfume and aroma houses work in similar microgram scales, especially if they're building a layered scent in roasted or tobacco notes.
With a lot of food flavors, people see the label, spot a chemical name, and worry about safety. This reaction is justified, and the food safety side has done its homework. The US FDA lists 2-Ethyl-6-Methylpyrazine as Generally Recognized As Safe (GRAS). Even so, flavorists always measure meticulously—precision turns out to be the only path to a great result. Adding too much ends up as a lesson in humility; I’ve seen cookie batches end up in the bin because one careless extra drop made everything taste like charred popcorn mixed with metal.
Beyond taste, lower doses reduce costs and keep the ingredient list short. Nobody wants to mask flavors artificially or hide behind excessive seasonings. Instead, thoughtful use allows the core taste of the food to come through, with a subtle roasted depth that doesn’t bulldoze everything else on the plate.
My own kitchen mistakes echo advice from pros: go low and slow. Always start at the low end of a suggested range, and taste-test at each step. For home use, blending a tiny drop in neutral oil dilutes the molecule, which helps avoid overdoing it. If working with a commercial scale, using calibrated micro-pipettes or standardized dilution solutions brings real consistency. Sensory panels help: a diverse group of tasters will catch “off” flavors that one overconfident food geek might miss.
For companies, reviewing scientific literature and supplier recommendations remains standard practice—most flavor manufacturers provide starting points for dosage based on years of data. But nothing beats in-house testing with real products, real people, and honest feedback.
Food and fragrance developers continue looking for bold, roasted, nutty top notes without falling into the trap of using too much pyrazine. As consumers demand cleaner labels and layered, authentic tastes, the lesson stays true: a light touch beats a heavy hand, every single time. That approach lets great flavors shine, without crowding out what’s real and good.
Walk into a bakery and breathe in that warm, toasted aroma—there’s a good chance science plays a role in shaping that experience. 2-Ethyl-6-Methylpyrazine, a mouthful of a name, pops up in a lot of those roasted, nutty, earthy flavors we enjoy in all kinds of foods. Chocolate, coffee, roasted nuts, even some beers, get a boost from this stuff. Some might stumble over whether this ingredient feels real or artificial, so the question “natural or synthetic?” doesn’t just live in food labs—it ends up at kitchen tables and grocery aisles too.
Chemically, 2-Ethyl-6-Methylpyrazine has a clear-cut structure. Nature puts together this formula during roasting, fermentation, and the Maillard reaction—the browning that happens in cooking meats, baking bread, or searing vegetables. These natural processes throw off dozens of tiny compounds, with this pyrazine among them. Some foods like roasted coffee or cocoa beans generate these flavors on their own.
Large food companies don’t always lean on nature to supply enough of these flavors, though. The amounts made in coffee roasting or chocolate making hardly stretch very far. Sourcing directly from raw foods would crank up costs and waste. For that reason, manufacturers build 2-Ethyl-6-Methylpyrazine from other basic chemicals in controlled lab settings, often starting from petrochemicals or plant-based feedstocks.
Folks worry that synthetic means fake or unsafe. It’s fair—eating something with a science-y name feels different than picking thyme fresh from a backyard planter. But the body can’t tell the difference at a molecular level; the structure remains the structure. In the U.S., the FDA checks these flavor molecules for safety. Europe requires strong labeling and traceability. Food science copies nature not to cut corners, but because nature doesn’t always scale up to modern demand.
Most home cooks and eaters want something real in their food—something that lines up with their values and health goals. A farmer’s market shopper searching for local honey wants clean labels and short ingredient lists. More folks read product packaging and scan for the catchwords “natural flavor” or “artificial flavor.” In practice, “natural” only applies if the flavor is physically extracted from plant or animal sources, not if it’s re-created molecule by molecule in a lab. This causes some confusion, with a lot of gray in between.
People keep asking for whole foods and minimally processed options. Companies that lean into transparency—sharing the real story about sourcing and production—build better trust. If a food label reads “artificial flavoring,” claiming honesty wins over heavy-handed marketing. On the regulatory side, clearer definitions for what counts as “natural” help. One simple move could be mandating detailed labeling, not just broad categories, so shoppers aren’t left second-guessing what sits in their snack bag.
For those worried about origins, sticking to single-ingredient foods or buying from brands that detail their flavor sources puts more control in the consumer’s hands. Home kitchens have a chance to celebrate bold, roasted flavors by using real coffee, cocoa, and toasted spices—and skipping the lab stuff altogether. That’s a reminder that food culture, science, and trust are tangled up, not separate parts of a recipe.
| Names | |
| Preferred IUPAC name | 2-ethyl-6-methylpyrazine |
| Other names |
2-Ethyl-6-methylpyrazine 2-Ethyl-6-methylpyrazin 2-Methyl-6-ethylpyrazine |
| Pronunciation | /ˈtuː ˈɛθɪl sɪks ˈmɛθɪl paɪˈreɪziːn/ |
| Identifiers | |
| CAS Number | [18218-50-5] |
| 3D model (JSmol) | `3Dmol.js?query=CC1=NC(=CN=C1)CC` |
| Beilstein Reference | 3203062 |
| ChEBI | CHEBI:142535 |
| ChEMBL | CHEMBL3210292 |
| ChemSpider | 153773 |
| DrugBank | DB14165 |
| ECHA InfoCard | 03dec5a6-2f97-4e29-8686-b16d5c8feb66 |
| EC Number | 211-579-2 |
| Gmelin Reference | 841636 |
| KEGG | C06578 |
| MeSH | D020961 |
| PubChem CID | 12997 |
| RTECS number | UY7475000 |
| UNII | Q5VQ1NKB2D |
| UN number | UN3267 |
| Properties | |
| Chemical formula | C7H10N2 |
| Molar mass | 122.17 g/mol |
| Appearance | Colorless to light yellow liquid |
| Odor | Nutty; Roasted; Popcorn |
| Density | 1.03 g/mL at 25 °C (lit.) |
| Solubility in water | slightly soluble |
| log P | 1.2 |
| Vapor pressure | 0.13 mmHg (25 °C) |
| Acidity (pKa) | 14.46 |
| Basicity (pKb) | 1.90 |
| Magnetic susceptibility (χ) | -64.5·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.5160 |
| Viscosity | 1.089 mPa·s (25 °C) |
| Dipole moment | 1.74 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 239.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | 37.2 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4247.8 kJ/mol |
| Pharmacology | |
| ATC code | '' |
| Hazards | |
| GHS labelling | GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. H319: Causes serious eye irritation. |
| Precautionary statements | P261, P264, P271, P272, P273, P280, P302+P352, P321, P362+P364, P501 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | Flash point: 99°C |
| Autoignition temperature | 320 °C (608 °F; 593 K) |
| Lethal dose or concentration | LD50 (oral, rat): 460 mg/kg |
| LD50 (median dose) | LD50 (median dose): 794 mg/kg (oral, rat) |
| NIOSH | USY008Q8ZV |
| PEL (Permissible) | Not established |
| REL (Recommended) | Not established |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
2,3-Dimethylpyrazine 2,5-Dimethylpyrazine 2,6-Dimethylpyrazine 2,3,5-Trimethylpyrazine 2,3,6-Trimethylpyrazine 2-Ethylpyrazine 2-Methylpyrazine 2-Ethyl-5-methylpyrazine |