Chemistry as a field rarely sits still. The late twentieth century introduced 2-Ethyl-4-Methylimidazole with a promise to shake up curing agents and polymer development. As research into imidazole derivatives picked up due to epoxy resin application demands, this compound found a foothold in new methodologies. Laboratories moved beyond basic cyclization of monomers; innovations crept in from pharmaceutical companies exploring antifungal properties and polymer plants chasing sturdier plastics. The connections between those projects still show in papers and patents today.
Chemists classify 2-Ethyl-4-Methylimidazole as a substituted imidazole, a five-membered heterocycle favored for its unique electron structure. In practice, you spot this compound in off-white crystalline or powder form. I remember opening the sample bottle and the faint musty odor stuck around a day. Its solid appearance betrays its high solubility, especially with polar solvents. Buying technical-grade products, labels read like a list of every place an adhesive finds use—from winding electrical coils to bonding high-strength composites.
With a melting range near 50–60°C, the substance feels waxy but does not melt at body temperature. The boiling point sits well above 250°C, so high heat is safe. Density falls around 1.05 grams per cubic centimeter, making it manageable for handling and transport compared with heavier aromatic amines. Chemically, that imidazole core reacts just as textbooks describe, with lone pairs on the nitrogen supporting base catalysis. Shelf stability depends on humidity; overexposure lets it clump up, which I learned firsthand after leaving a bag too loosely sealed.
Industrial users demand clear numbers. Purity usually draws the line at 98% or above. Impurities drop below 1%, with strict limits on moisture and heavy metals set by manufacturers. Labels carry hazard codes, GHS pictograms, and warn about direct skin or eye exposure. Chemical Abstracts number 931-36-2 distinguishes this compound; trade names and lot data hit every package. Datasheets emphasize exact melting points, appearance, and assay results to build trust batch-to-batch. Every lab worker checking in new stock knows to scan these for changes, since small shifts in the physical data alter how it behaves later in synthesis.
The basic synthetic route starts from glyoxal, ammonia, and corresponding aliphatic aldehydes, all meeting under reflux conditions. Lab protocols sometimes swap reagents to tweak selectivity or yield, but the ring closure remains central. Yields climb following optimization, especially under anhydrous conditions or in polar aprotic solvents. Scale-up introduces heat recovery to manage exothermal peaks, and even minor shifts in temperature profile can throw off purity. Experienced operators keep careful logs and rely on back-titration and chromatography to monitor conversion. For many mid-sized chemical companies, this synthesis process became a badge of quality in the epoxy agent market.
The imidazole framework opens many doors. Ring alkylation creates whole families of curing agents, each fine-tuned for specific resin systems. Reactivity as a nucleophile enables acylation and etherification, but the stable core resists outright breakdown under most conditions. Technicians exploring crosslink density often graft additional functional groups onto the imidazole backbone, using mild acid or base catalysis to ensure selectivity. I’ve seen talented chemists pull off clean substitutions that turn the raw material into specialty intermediates for agrochemicals or advanced electronics adhesives.
Chemical language is rarely straightforward. Common synonyms include 2-Ethyl-4-methyl-1H-imidazole and EMI. Older catalogs might mention EMZ or Ethylmethylimidazole, revealing the sometimes inconsistent trade landscape. In various markets, branded packages show proprietary formulations, with names like Curezol EMI or Novacure. Any ordering department handling multiple suppliers needs a strong familiarity with synonyms and alternate names, since one missed letter can pull up the wrong safety documentation or technical sheet, causing problems downstream.
Safe use demands attention. Dust from fine powders irritates both respiratory tracts and skin, making fume hoods and gloves basic protocol. Eye contact brings out full eyewash procedures, since stinging and redness are not uncommon on even brief exposure. Chronic overexposure can sensitize skin, so long sleeves, fitted goggles, and even negative-pressure workstations come into play in heavy manufacturing. OSHA regulations kick in at the bulk handling stage, with additional controls for waste management and air monitoring. Storage best practices favor sealed, dry containers housed away from acids or oxidizers, because incompatible mixing can drive runaway reactions.
I have watched 2-Ethyl-4-Methylimidazole transform resin formulations across sectors. Its main draw lies in accelerating epoxy cures, forming bonds at much lower temperatures than traditional amines. Electronics manufacturers use this property in printed circuit production, slashing time and energy use on assembly lines. Wind turbine blades, aerospace composites, and automotive structures all benefit from the mechanical properties and adhesive strength this curing agent imparts. Researchers occasionally explore its potential in corrosion-resistant coatings, but the focus stays on resins because that’s where both performance and efficiency shine brightest.
Research labs churn out variants, sometimes pursuing thermal latency for one-pot systems, other times looking to slash toxicity without sacrificing speed. Published studies examine the relationship between imidazole structure and cure kinetics. Tech companies push for greener synthesis, reducing waste and embracing solvents with low environmental impact. Pharmaceutical researchers ran trials in antifungal drug candidates, drawing on imidazole’s medicinal legacy, but application here remains limited by tight toxicity profiles. Some universities run graduate projects pairing this compound with next-generation bio-based epoxies, searching for ways to pair performance with sustainable supply chains.
Tox data shows a mixed bag. Acute exposure doesn’t reach dramatic levels found in more toxic organics, but irritation bothers noses and eyes quickly. Older studies flagged sensitization risks—lab animals developed contact dermatitis after repeated use, and handling guidelines evolved to keep skin exposure low. No solid links to cancer or reproductive toxicity emerge from published datasets, but chronic inhalation studies remain ongoing, especially given rising interest in workplace safety. Environmentally, breakdown is fairly rapid under aerobic conditions, although companies treat waste as hazardous to avoid accidents. Anyone claiming the compound is completely harmless hasn’t spent enough time in handling suits or monitoring fume hoods.
Demand for materials that cure fast, perform under stress, and keep costs reasonable pushes industry toward compounds like 2-Ethyl-4-Methylimidazole. As energy costs force factories to rethink every heating step, imidazole-cured epoxies look increasingly attractive. Sustainability projects chase new routes from renewable feedstocks, and digitalization amplifies quality tracking in every batch. I see more labs rolling out advanced analytics on toxicity and environmental impact, with young chemists eager to push this core structure into bio-based composites or even medical devices. Companies willing to innovate with both setup and safety will carve out steady business, since fast, reliable bonding seldom goes out of fashion in industry or research.
Think about building a bridge, making electronics, or putting together the walls of your house. The glue, sealants, and coatings used in these jobs must handle heavy loads, high heat, or lots of moisture. Behind the scenes, 2-Ethyl-4-Methylimidazole stands out as one of those small molecules that flips the switch from “sticky mess” to “permanently bonded.” In my own experience smoothing epoxy into cracks in an old garage floor, the difference between a solid permanent patch and a weak repair depends a lot on how those chemicals set—and this is where compounds like this imidazole become essential.
Most people run into epoxy in hardware stores or crafts, but for industries, it’s the backbone of strong adhesives and coatings. The catch with epoxy is that it needs a “curing agent”—something to trigger the hardening and cross-linking that makes the material actually useful. One of the best things about 2-Ethyl-4-Methylimidazole comes from how quickly and completely it reacts with epoxy resins. Finished parts can face high heat or load without cracking, which is critical for cars, aircraft, or electronics. Long-winded curing processes slow down production and increase costs, so a reliable agent like this helps factories skip endless waiting and get quality products out the door.
Electronics get smaller and faster every year. Their tiny circuits must stay protected against moisture, dust, and rough handling. Using this imidazole in circuit board assembly strengthens the layer that shields those fragile copper lines. It’s not glamorous, but without this kind of chemistry, devices would short out or corrode far quicker. I’ve watched repair techs troubleshoot faulty gadgets, and the difference often comes down to whether the internal coatings hold up. With reliable curing, the gadgets we count on don’t fizzle every time the weather shifts.
For construction, marine, or even do-it-yourself repairs, coatings often do much more than look pretty. They stop water from creeping in, block chemical spills, and keep surfaces from wearing out early. Here, the imidazole improves both the speed and quality of the hardening process. Surfaces get back into use faster, which matters a lot if you’re running a factory floor or finishing a public pool before summer hits. My own brush with patching concrete before a family gathering drove home the value of products that harden quickly—and stay that way through heat, foot traffic, and the occasional spilled drink.
Strong chemicals often raise real safety and environmental concerns. Imidazoles, in small amounts, offer the durability needed without the heavy hand of harsh catalysts that foul up air or linger in soil. Manufacturers have pushed towards using less-toxic, more efficient chemicals, both for worker safety and long-term habitability. There’s plenty of room to keep improving, trading out older, less-friendly curing agents for versions that balance strength and eco-friendliness.
Every step towards more sustainable chemistry means better health for workers, safer homes, and less damage downstream. Alternate curing systems already pop up, but matching the performance and reliability of 2-Ethyl-4-Methylimidazole isn’t easy. Out in the real world, the goal is to keep the strong bonds and cut the environmental baggage, making sure the things we build last, but without making the planet pay the price.
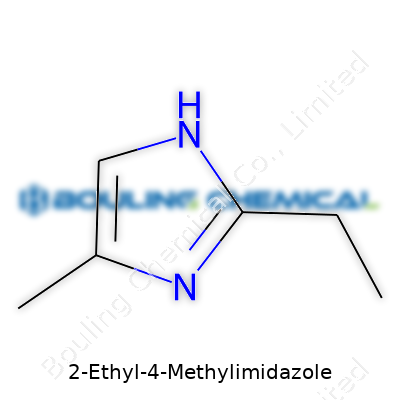
Working in labs and on factory floors, I came to see certain chemicals over and over. 2-Ethyl-4-Methylimidazole is one of those. Its name sounds clunky, but it packs clarity for anyone who needs it to solve real problems. The structure rests on an imidazole ring. Off that ring, there’s an ethyl group at carbon 2 and a methyl group hanging from carbon 4. Chemists draw it as a five-member ring, two nitrogens placed together, with the bulky side-chains giving it extra personality.
This structure—look it up as C6H10N2—matters more than its bland label might suggest. The CAS number, 931-36-2, acts as a unique fingerprint for tracking, safety, and trade. Having a CAS number simplifies ordering, safety data retrieval, or customs paperwork. In labs, you never want to gamble on a lookalike compound; a single digit’s difference can mean sports car or lemon.
During my time mixing epoxy batches, 2-Ethyl-4-Methylimidazole often showed up as a curing agent. Its side chains balance reactivity and shelf-life. Too fast, and you never get time to mix or apply. Too slow, and the shop floor bottlenecks every job. This molecule strikes a productive rhythm. That’s not some lucky accident. Chemical tweaks, like adding a methyl here or ethyl there, shape the way two-part systems set up or resist heat.
If you care about circuit board reliability, you care about this compound’s structure. Inspections in electronics assembly exposed plenty of circuit failures from poor curing. Using a dialed-in imidazole means less downtime and lower warranty returns. Small businesses watched the difference on their bottom lines. A single missed batch builds more headaches than most realize until it lands on someone’s desk.
Chemical names and numbers may seem academic, but safety officers know them as lifelines. 2-Ethyl-4-Methylimidazole isn’t especially exotic in handling, but it still calls for gloves and eye protection. MSDS sheets list the CAS number to ensure teams use the right measures for spills or exposures. No shortcuts add up to unnecessary ambulance calls—for employers and workers alike, that's not money or trust well spent.
Supply chain glitches put the spotlight on identifiers like CAS numbers. Spot shortages during a busy year can push folks to alternate suppliers or resellers. A quick reference of that 931-36-2 number keeps the substitute check easy—no guesswork, less risk of costly mistakes due to mislabeled drums. For importers, that number proves crucial for shipping documents and compliance in different regions.
Quality control teams sometimes gripe about variability in epoxy results. From my own troubleshooting, I noticed off-brand versions sometimes sneak extra water or mismatched isomers. Clear labelling with CAS numbers and verified certificates blunt these risks. Suppliers have incentive to prove origin and batch consistency if their buyers ask firmly. That’s a habit worth spreading, especially as more manufacturing moves to global networks with heavy expectations.
Amid innovation pushes and regulatory tightening, industry finds itself juggling cost controls with transparency. Forward-looking companies invest in supply audits and stronger documentation—no different than the lot tracking systems found in pharmaceuticals. As demand for robust, reliable polymers grows, so does the need for traceable raw materials like 2-Ethyl-4-Methylimidazole. Reliable structure and confirmed CAS registry set the stage for less drama and smoother technical progress.
I’ve seen more problems caused by bad storage than by anything else in product management. One time, a warehouse stacked cartons of food supplements directly by the south-facing window. The heat cooked the product inside the boxes. Some supplements melted and stuck together; the labels peeled off in humid corners. That shipment triggered a mess no one wanted—returns, refunds, and frustrated customers. The lesson came loud and clear: how a product sits on a shelf makes all the difference once it’s taken off that shelf.
Heat warps plastics, degrades medicine, and changes the taste of food. Cold can turn liquids into sludge and crack glass containers. Check the label. If it says room temperature, don’t ignore it. Warehouses heat up like ovens in July. At home, a back-of-the-cupboard spot away from your stove works best. Pharmacies and grocery stores keep coolers for a reason. It isn’t overkill—heat and cold change what’s inside those bottles and boxes in ways most folks can’t see until it’s too late.
Moisture sneaks in unnoticed and ruins products fast. Powdered items attract water and clump together. Mold creeps into paper packaging left in damp areas. If you live somewhere humid, you probably own a bag of rice just to stop the salt shaker from turning into a brick. Silica gel packets thrown into boxes or taking steps to keep things off a basement floor go farther than most people realize. Even coffee stored in the wrong spot goes stale and takes on odd flavors.
Not all damage is obvious. Sunlight fades colors, breaks down chemicals, and spoils vitamins. Some chemical products get weaker just sitting under bright lights. There’s a reason dark glass bottles protect some prescription drugs. The extra penny spent on the right container saves money and headaches down the line. Retail shops with big glass windows often see their display items age fast. Turning on a light for three days can change a product’s color or even reduce potency.
Packing products like sardines often leads to dents, leaks, and tears. Canned goods collapse bottom cans if stacked too high. Bottles stored on their sides leak if caps loosen over time. Shelving with space for airflow helps everything stay fresher. Some products give off gases as they age—fruit releases ethylene and ripens everything near it. Pretty soon one spoiling item turns into a shelf of waste. Smart handling involves regular checks, giving cartons space, and rotating older stock forward.
Most issues I treat as avoidable. If the handling instructions say “keep dry” or “store below 25°C,” someone put that there for a reason. A pen, a sticky note, or even tape can flag sensitive products. Clear labeling cuts confusion. Staff in a rush sometimes grab the wrong item, so that warning sticker might save a week’s worth of business trouble. I always tell people: if you see odd symbols or words like “fragile,” trust them.
I’ve found that keeping a regular schedule to check storage areas prevents half the headaches. A quick walk-through every week beats sifting through a surprise mess at stocktake. Even at home, glancing at the pantry every Sunday keeps things from slipping through the cracks. Commercial storage operators use inventory rotation plans—first-in, first-out—so nothing sits untouched while newer items shift forward. At the end of the day, care in storage and handling shows in fewer complaints and less waste.
Names like 2-Ethyl-4-Methylimidazole rarely show up in daily conversation, but for people working in manufacturing, electronics, or chemical plants, the risks tied to it feel real. This chemical pops up as a curing agent in epoxy resins—the stuff that holds wind turbines, car parts, and circuit boards together. Small molecule, big impact. It’s used because it brings strength and heat resistance. But as soon as I hear a synthetic chemical enters a workspace in high volumes, alarm bells ring for good reason.
I remember my own first day walking into a resin lab: the nose-puckering smell, the warning symbols pasted everywhere. It made an impression. Sharing space with powerful chemicals calls for more than common sense. You want to know what you’re handling, especially with compounds like this one. According to the European Chemicals Agency (ECHA) and OSHA, 2-Ethyl-4-Methylimidazole causes skin and eye irritation and can cause respiratory discomfort if you breathe in dust or vapor. It won’t explode, but it can sting, burn, or start an unpleasant cough. That’s enough reason not to treat it like sugar or table salt.
You don’t have to navigate these dangers alone—or ignore them. Proper labeling and storage help. So does good ventilation. I learned the hard way how much difference gloves make when handling unknowns: a quick splash can leave a burning patch on unprotected skin. The safety data sheets tell you exactly what gear you need: gloves that resist chemicals, splash goggles, maybe a face shield in a busy setting, and coveralls if there might be spills.
Nobody expects to get sick from going to work or running a commercial printer. Still, years of breathing in low doses of dust, or brushing a wrist across contaminated surfaces, stack up. Slow, repeated exposure sometimes leads to sensitivity or allergies—something you might not notice until it’s too late. That sort of thing isn’t dramatic, but it hits home for people who use their hands and lungs every day.
Laws set exposure limits for a reason; they’re built from mistakes made decades ago, when the long-term health costs came out in lawsuits and studies. Companies can minimize risks by investing in fume hoods or local exhaust systems, making sure safety data gets updated and shared, and running regular staff training. That sense of control—knowing exactly where your chemicals come from and how they’re being used—means trouble gets caught early.
I’ve seen managers slack off, arguing cost or convenience, until inspections rolled through or someone reported a rash. Nobody wants to be the squeaky wheel, but open conversations matter. Staff should be encouraged to flag concerns without backlash. Regular check-ins, up-to-date training, and real hazard communication turn a workplace from an accident waiting to happen into somewhere safer for everyone.
No one expects perfection. Chemicals like 2-Ethyl-4-Methylimidazole have a place in industry, but respect and diligence need to match their potential hazards. Prevention always costs less than medical bills or lost workdays. Giving workers the right tools and the freedom to speak up about risks makes all the difference.
Chemists who’ve handled 2-Ethyl-4-Methylimidazole know its reputation: a versatile curing agent, tough enough for modern epoxy resins, adhesives, and electronic encapsulants. Sitting at a workbench, you learn to trust specifics, not generalities. Somewhere in the linoleum glare of the warehouse or the hum of lab hoods, that bottle from the supplier arrives—and the first glance isn’t for the color or the label but for the certificate of analysis. What purity level did they use this time?
In practice, specifications matter because results depend on them. Purity for 2-Ethyl-4-Methylimidazole, as received from major vendors, typically falls in the 97%–99% range. Technical grade products hover at the lower end, while those destined for more demanding electronic or pharmaceutical purposes touch the upper end, sometimes showing 99%+ on the spec sheet. Buyers in advanced sectors push the bar higher each year. Low-purity chemicals introduce unknowns—impurities risk erratic reactivity, color shifts, and variations batch to batch. Some specialty applications place orders only after stipulating GC-MS or HPLC trace impurity profiles showing what comprises the remaining percentage.
I’ve seen labs frustrated by issues that trace back to lax purity standards. Anyone running formulations using imidazoles knows that 2% impurity can throw off curing times, lower electrical resistance, or weaken the ultimate bond strength. Not all of these contaminants affect performance the same way, so pure numbers don’t tell the whole story. But what sneaks in matters: water, unreacted starting materials, and related side-chain imidazoles all play their part. High-purity product, confirmed by modern analyses, reduces the risk of headaches down the line.
Problems scale up with production. A bad run of an epoxy or a batch of electronics with micro-cracks usually costs far more than paying extra for higher purity material upfront. I’ve seen one project stall for months because a supplier shipped a drum that nearly met the spec but hid trace amines inside—no one checked until curing schedules collapsed under real-world stress. These aren't flukes. Over and over, the devil is in the details—the minor components tagging along with the main show.
End-users who care about reliability demand certificate-backed proof. They look past “minimum 97%” toward the next line: what’s in that extra 3 percent? Reputable suppliers include tight limits for water, volatile bases, and color number—sometimes even optical rotation. Folks serious about scaling or about regulatory submission (think pharma grade or certain electronics) add their own acceptance criteria, demanding documentation like residual solvent levels or confirmation by NMR. Some request third-party analyses.
There’s a role for both industry watchdogs and customers to push tougher guidelines. I’d argue that buyers need to insist on seeing not just purity by titration, but impurity breakdowns. This should move beyond the certificate—random spot checks, comparison across batches, and even return of out-of-spec material. Risk tolerance is shrinking: so must the allowance for “good enough” purity. Labs and manufacturers should partner up to specify not just the main number, but the details underneath it—a step toward fewer headaches, safer production runs, and a final product that performs as promised.
| Names | |
| Preferred IUPAC name | 4-Ethyl-2-methyl-1H-imidazole |
| Other names |
2-Ethyl-4-methyl-1H-imidazole 2-Ethyl-4-methylimidazol 4-Methyl-2-ethylimidazole |
| Pronunciation | /tuː ˈɛθɪl fɔːr ˈmɛθɪl ɪmˈɪd.əˌzoʊl/ |
| Identifiers | |
| CAS Number | 931-36-2 |
| Beilstein Reference | 136446 |
| ChEBI | CHEBI:85143 |
| ChEMBL | CHEMBL405867 |
| ChemSpider | 63554 |
| DrugBank | DB11268 |
| ECHA InfoCard | String: 100.018.600 |
| EC Number | 208-759-1 |
| Gmelin Reference | 1632326 |
| KEGG | C21121 |
| MeSH | D000081223 |
| PubChem CID | 14637 |
| RTECS number | MK4460000 |
| UNII | Z4FD2HD13D |
| UN number | UN3337 |
| Properties | |
| Chemical formula | C6H10N2 |
| Molar mass | 123.18 g/mol |
| Appearance | White to yellowish crystalline powder |
| Odor | amine-like |
| Density | 0.99 g/cm³ |
| Solubility in water | Soluble |
| log P | 0.38 |
| Vapor pressure | 0.0000827 mmHg at 25 °C |
| Acidity (pKa) | 7.7 |
| Basicity (pKb) | 6.59 |
| Magnetic susceptibility (χ) | -65.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.496 |
| Viscosity | 14 cP (20 °C) |
| Dipole moment | 2.76 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 233.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -24.57 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3805 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed or in contact with skin. Causes skin irritation. Causes serious eye irritation. May cause an allergic skin reaction. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS05, GHS07, GHS08 |
| Pictograms | GHS05,GHS07 |
| Signal word | Danger |
| Hazard statements | H314: Causes severe skin burns and eye damage. H302: Harmful if swallowed. H332: Harmful if inhaled. |
| Precautionary statements | P261, P264, P280, P301+P312, P305+P351+P338, P337+P313, P405, P501 |
| NFPA 704 (fire diamond) | 1-3-0 |
| Flash point | 86°C |
| Autoignition temperature | 385°C |
| Lethal dose or concentration | LD50 Oral Rat 850 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral-rat LD50: 960 mg/kg |
| NIOSH | WSH6698000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | REL (Recommended Exposure Limit): 0.1 mg/m³ |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Imidazole 2-Methylimidazole 4-Methylimidazole 2-Ethylimidazole 1-Methylimidazole 2-Phenylimidazole 2-Ethyl-5-methylimidazole 2-Propylimidazole |