2-Ethyl-3-Methyl Pyrazine didn’t leap into our lives by accident. Back in the early 20th century, chemists chasing the essence of roasted and nutty flavors stumbled across this compound in the lab, mostly by analyzing how heat transformed amino acids in cooked foods. Researchers kept finding derivatives of pyrazines in coffee, roasted grains, and nuts. The 1970s brought real focus as food and fragrance chemists began to separate, identify, and reproduce individual pyrazines. I remember reading that European flavor houses led efforts to isolate and commercialize this molecule. Nowadays, the compound stands as a staple for crafting aromas that bring foods to life, all rooted in the momentum those early scientists built.
2-Ethyl-3-Methyl Pyrazine belongs to the pyrazine family, famous for shaping the scents and tastes that define many foods. It often carries a roasted, nutty, earthy signature—think of the smell just as coffee beans hit the grinder, or when peanuts emerge from the oven. Its robust aroma packs a punch even at low concentrations, which gives formulators the power to tweak flavors without overwhelming the recipe. The industry tends to source this molecule both synthetically and through extraction from roasted foods, but controlled lab production dominates due to purity demands and cost. Its appearance? Clear, colorless to pale yellow, slightly oily, with an unmistakable scent that experienced noses spot right away.
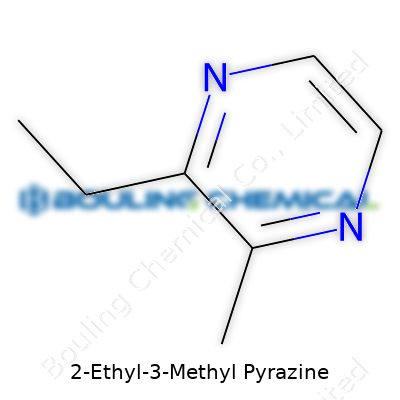
2-Ethyl-3-Methyl Pyrazine comes as a liquid at room temperature, with a boiling point in the 160–170°C range, and a melting point well below zero. With a molecular weight of 122.17, and the formula C7H10N2, the compound’s small, rigid structure lets it slip into volatile headspaces—exactly where aroma specialists want it to go. Its density sits around 0.98 g/cm3. Solubility tilts toward organic solvents and oils, but water mostly shrugs it off. The compound resists breakdown under mild heat, which matters in baking or roasting applications. Pyrazines rarely act alone, though; in practice, the complex interplay with aldehydes, ketones, and other pyrazines forms the backbone of toasted, savory smells.
Pure 2-Ethyl-3-Methyl Pyrazine products often clock in at 98% or higher assay by GC, and suppliers have to prove their claims through traceable certificates of analysis. You’ll usually see it labeled by CAS number 15707-23-0, with UN numbers and hazard warnings reflecting moderate irritant properties. Regulatory agencies—FDA in the US and EFSA in Europe—require its declaration in ingredient lists for food applications, and industry players watch purity markers like optical rotation and residual solvent content. Packaging ranges from tiny amber bottles for R&D to bulk drums for manufacturing lines, all meeting strict standards to avoid contamination.
Synthesizing 2-Ethyl-3-Methyl Pyrazine starts from simple building blocks—usually ethylamine and methylglyoxal—put together under controlled heating with catalysts. Supervision and careful adjustment keep byproducts out, since any off-note in a flavor ingredient quickly sinks a product line. Some labs use recycled side streams from roasted food processing to reclaim natural pyrazines, though chemical synthesis dominates. Upscaling from the bench to the plant isn’t trivial; batch consistency, solvent recovery, and waste stream management often turn into bigger challenges than the chemistry itself. Researchers keep chasing greener and leaner methods, but for now, the direct synthetic route holds its ground.
The core pyrazine ring stands up to all sorts of tough conditions, so direct modifications rely on selective substitutions rather than harsh treatments. Halogenation or selective reduction opens doors to making more specialized aroma molecules, although too much tinkering erases the characteristic aroma. Nitration or additional alkylation can extend the flavor palette but often muddies the profile. Some flavorists experiment with mild oxidation to soften or round out the aroma, producing subtle nutty or earthy undertones. Each tweak comes with trade-offs: higher cost, stricter controls, or new safety labels.
Tracking this molecule through the marketplace calls for a sharp eye, since names vary by supplier and regulatory context. You’ll find synonyms like 3-Methyl-2-Ethylpyrazine, or simply “Nutty Pyrazine A” among flavorists. European labels sometimes list it as 2-Ethyl-3-Methyl-1,4-Diazine. Some big brands market it under trade names meant to evoke warmth, baking, or roasted themes, but the CAS number always anchors the true identity on paperwork and export declarations.
Pyrazines in liquid form tend to be skin, eye, and respiratory irritants, so plant operators wear gloves and goggles during handling. Storage in cool, dry, and ventilated rooms reduces accidental vapor buildup, as inhaling concentrated fumes stresses airways. Food safety teams set strict intake limits, far below levels that cause harm, drawing on animal and human data. Workers follow spill and cleanup protocols tailored to aromatic organic liquids; good ventilation, absorbent materials, and proper disposal cut fire and health risks. Training refreshers keep everyone aware that aroma chemicals might be tasty in tiny drops but belong nowhere near open flames or sensitive skin.
Food industry leads the pack, adding this molecule to chocolate, coffee, roasted nuts, cereals, baked snacks, and even some plant-based dairy mimics. It pops up in pet foods, imparting life to bland kibble. Fragrance houses blend it into fine fragrances and air care, especially for “freshly brewed coffee” room sprays. Tobacco and vaping sectors use small amounts to engineer smooth, consistent aromatic profiles. My experience with chocolate manufacturers shows that even a few parts per million can transform mass-produced goods into crave-worthy treats. Some pharmaceutical formulas tuck in pinches for masking bitter tastes, but regulatory hoops here run tighter.
Academic labs dig into pyrazine biochemistry, figuring out pathways in roasted and fermented foods, hoping to boost natural yields or develop new variants. R&D teams look for more efficient, less resource-heavy synthetic routes, often focused on lowering waste and power demands. There’s a lot of interest in natural extraction from food processing sidestreams, especially where food waste management doubles as a feedstock opportunity. Analytical chemists refine detection techniques, since trace spikes or drops have a big impact on food quality control. Teams push into digital modeling, predicting flavor interactions for new recipes—a mix of tradition and tech that keeps the field fresh.
Animal studies at high doses—orders of magnitude above food use—suggest mild acute toxic effects: skin irritation, short-term metabolic changes. Rodent data backs up current regulatory exposure limits, with digestive and metabolic clearance happening rapidly at normal dietary levels. Chronic exposure research focuses on cumulative intake, especially in populations with high processed food consumption, but available data suggests safety below legal limits. Ongoing studies watch for developmental or neurological impacts. Labs check for potential breakdown products during heating, making sure no nasty surprises creep into finished products or fumes.
Plant-based foods and health-conscious consumers push producers to rethink how 2-Ethyl-3-Methyl Pyrazine gets sourced and processed. Companies race toward natural, upcycled, or “green chemistry” versions, aiming for labels like “naturally derived” or “sustainably sourced.” Digital recipe modeling and high-throughput screening speed up the hunt for new flavor pairings, sometimes revealing synergies with less familiar pyrazines or flavor boosters. Synthetic biology might shake up the field, too—microbes tailored to churn out pyrazines during fermentation could lower costs and environmental impact. Regulatory agencies tighten purity rules and force more traceability, especially for exports. As the food, fragrance, and sustainability industries evolve, this molecule isn’t fading from the spotlight.
Pour a cup of coffee, break open a chocolate bar, or get a whiff of roasted nuts in the kitchen, and you’ve likely come across the work of 2-Ethyl-3-Methyl Pyrazine. This molecule doesn’t grab headlines, yet it shapes the way our food smells and tastes. As someone who’s worked in a bakery and spent years tasting new products in grocery labs, I spot this compound every time I read labels or flavor profiles on new snacks.
Walk down the snack aisle, and notice that rich, deep aroma from roasted chips or savory crackers. That signature smell and flavor often owe a lot to pyrazines, especially 2-Ethyl-3-Methyl Pyrazine. Manufacturers sprinkle it into seasonings and mixes to recreate the scent of cooked, toasted, or nutty foods. Without it, the crunch from a handful of peanuts or a bite of roasted coffee beans would fall flat. I still remember my first time doing a side-by-side taste test for seasoning blends. One sample—lacking this pyrazine—tasted bland, almost empty. Add it back in, and the roasted flavor returned instantly.
Chocolate companies lean on 2-Ethyl-3-Methyl Pyrazine to deliver that mouthwatering cocoa aroma, especially in milk chocolate or products that get a nutty boost. Roasted coffee relies on this compound, too. It comes about naturally during the Maillard reaction—the process that browns foods and gives them much of their flavor. Roasters adjust their process so these molecules form at just the right time, then lock in the best scent.
A walk through the world of artificial fragrance reveals how pyrazines show up in candles, perfumes, and diffusers. Home fragrance brands appreciate its earthiness, which compliments both woodsy colognes and food-scented candles. Its presence can turn a synthetic vanilla candle into something that actually smells “baked” instead of fake or powdery. Any home cook who’s tried to capture the smell of browning onions or roasted almonds in a bottle knows how tough this is—this molecule helps bridge that gap.
Fast food chains and snack companies expect each batch of product to taste and smell identical, whether customers are in Texas or Taiwan. 2-Ethyl-3-Methyl Pyrazine gives them a dependable way to do this. That’s not a bad thing—consistency also means no big let-down for people who buy their favorites again and again.
As more shoppers read ingredient labels, concerns about “artificial” ingredients pop up. It’s worth noting that this particular pyrazine occurs naturally in food, yet commercial versions often come from factories. Regulatory agencies, like the FDA in the United States, have reviewed its use in reasonable amounts, listing it as “Generally Recognized as Safe.” Over the years, I’ve watched product developers switch to more “natural” flavorings under pressure from shoppers, but the chemistry remains the same. The real question is whether companies tell the truth about it and avoid stuffing foods with unnecessary extras.
Sustainable food scientists are working on techniques to coax these same flavors from fermentation or other natural processes, using fewer chemical shortcuts. They hope to deliver the same sensory punch with less environmental impact. If you check new products in stores, you’ll already see labels boasting “naturally fermented flavors” as an answer to concerns about artificial additives. Products made this way may cost more, but at least shoppers get some peace of mind—and still enjoy the same bold taste.
Open a bag of roasted peanuts or a box of toasted cereal, and the first whiff often teases something nutty, familiar, and almost savory. That’s the calling card of 2-Ethyl-3-Methyl Pyrazine — a molecule that deserves more than its clunky scientific name. This compound usually shows up in places where roasting or toasting does wonders. In my own kitchen, browning nuts sometimes fills the air with a pleasant, warm scent that’s hard to pinpoint, and this pyrazine quietly plays a big part.
Some flavors remind people of home, warmth, or simple comfort. 2-Ethyl-3-Methyl Pyrazine fits that bill. Its aroma lands solidly in the roasted peanut or popcorn ballpark, strong enough that even in tiny amounts, it stands up and gets noticed. Scientists call this compound a “potent odorant” for good reason. At concentrations as low as one part per billion, its scent starts to emerge. The flavor profile usually draws out nuttiness, dryness, and a toasted edge, which works in both sweet and savory foods.
Foods using this molecule often sell nostalgia as much as taste. Think of peanut butter, corn chips, or coffee. Food scientists know the Maillard reaction — that browning magic — sparks the creation of pyrazines like this one. In factories and home stoves alike, a little heat goes a long way. I’ve spent time testing home-roasted coffee, and every batch that steps beyond light roast brings out more of these earthy, hearty notes. The aroma doesn’t just announce itself — it can linger, sticking in your memory long after the food cools off.
Most people never see 2-Ethyl-3-Methyl Pyrazine on an ingredient label, but it pops up often, especially anywhere “natural flavors” show up. It’s not just in snacks, either — grain-based spirits and roasted malts in beer pull it in as well. Japanese whisky, for instance, leans on barrel toasting, which boosts this nutty layer and rounds out the bite. Finding ways to use pyrazines artfully separates mediocre food technicians from the flavor wizards. It’s a tool for consistency and a shortcut to rich flavor, but like good salt, a heavy hand ruins everything.
Every time a food company builds new products, there’s a temptation to crank up these flavors for maximum impact. Synthetic versions get tossed into processed snacks, soups, and sauces. I’ve noticed a risk, though — too much, or paired with off-putting additives, and the whole thing tastes artificial or stale. Consumers notice. Transparency in flavor engineering could build more trust, since foodies want to know what’s in their food and why it tastes a certain way.
Studies keep piling up showing pyrazines don’t just add flavor. They trigger psychological responses, conjuring comfort and satisfaction — and sometimes trick the brain into thinking a food is richer or more satisfying than it really is. Researchers at the German Institute of Food Chemistry nailed down the idea that people pick up on these nutty, earthy notes even when they don’t expect them. That’s one more reason chefs, distillers, and snack makers keep coming back to compounds like this, balancing them with the bigger flavor picture.
Understanding chemicals like 2-Ethyl-3-Methyl Pyrazine helps everyone, from home cooks to flavorists, make smarter, more ethical choices. Clean labeling, transparency, and a little restraint mean we all get food that’s both tasty and true to its roots. Every time someone bites into a roasted snack or sips on a complex whisky, there’s a story in that aroma — a story worth telling and protecting.
Most people grab a snack and rarely take the time to scan the ingredient list. But let’s talk about one name that sometimes pops up: 2-Ethyl-3-Methyl Pyrazine. That name doesn’t sound like something you’d sprinkle on your morning toast, yet it lands in the "flavoring" column of some processed foods. It brings a nutty aroma; think roasted peanuts or cocoa. So, what’s the real story behind this food additive, and can we trust it to stay safe in our meals?
Food science has tackled many flavors, both natural and lab-made. 2-Ethyl-3-Methyl Pyrazine comes from a family of compounds often found naturally in roasted foods. Chemists learned to make it in the lab. In small quantities, this molecule adds a roasted kick to snacks, chocolates, and bakery treats.
The safety of additives always presses on people’s minds, since lab-made ingredients still carry a long shadow. In the US, the Flavor and Extract Manufacturers Association (FEMA) reviewed this one, and the FDA put it on their GRAS (Generally Recognized As Safe) list. Basic toxicity tests showed no alarms with usual food-level exposure. The European Food Safety Authority (EFSA) gave a similar green light, pointing to low risk when people eat ordinary amounts.
It’s easy to see these official stamps and relax. In real life, science keeps moving, and safety stories aren’t always finished. Very few people actually eat one additive in isolation. Diets pile up loads of different chemicals, and studies rarely track such combinations over decades. Plus, most testing looks for short-term effects, missing the subtler, slower risks.
A handful of animal studies reached for extreme amounts far above what you see in an average diet. They found little evidence of harm at food-realistic doses, but gaps remain. Almost nobody independently tracks the long-term impacts of eating tiny bits of many lab-made flavors for an entire lifetime. So, trust in GRAS means leaning on the weight of probability and the cautious pace of science, not total certainty.
The bigger challenge lies in how these “safe” molecules reshape the food industry and our personal choices. Food manufacturers reach for consistent, shelf-stable flavors to hook repeat customers. Instead of roasting peanuts or brewing real chocolate, they settle for flavorings that often cost less and guarantee the exact same taste. People end up chasing familiar flavors, sometimes forgetting what real food tastes like, or how much processing shaped that morning breakfast bar.
On the consumer side, choice isn’t just about health—it's also about trust and taste. Each new chemical in food erodes a bit of that trust, especially if it crowds out the simple, familiar ingredients from past generations. People want to know what goes in their bodies, and they deserve clear labels and honest stories from food companies.
The best path forward starts with transparency. If 2-Ethyl-3-Methyl Pyrazine pops up in cookies or trail mix, the label should say so in plain language. Food makers should share not only the name, but the reason for using it, and what it actually adds. Scientists and regulators need to keep checking for new data, not just rubber-stamping old decisions. As customers, we can push brands to return to simpler ingredient lists and back that up by choosing whole and familiar foods more often.
Anyone who’s ever cracked open a freshly roasted bag of coffee, or taken a bite of a toasted nut mix, has experienced the magic of flavor compounds like 2-Ethyl-3-Methyl Pyrazine. This little molecule punches above its weight, delivering those rich, roasted, nutty notes that make so many foods crave-worthy. In flavor formulation, it's almost like a secret weapon.
Too much of a good thing quickly becomes a bad thing, though. With 2-Ethyl-3-Methyl Pyrazine, it's easy to tip the scale from subtle depth to overpowering bitterness. Through trials, and wandering through ingredient lists for years, I've learned this compound works best at extremely low concentrations—think parts per million. For food flavors, industry practice hovers between 0.01 ppm to around 3 ppm, depending on what you're making.
Imagine trying to catch up with a friend over coffee, but your face keeps wrinkling because the aroma hits you like an espresso to the nose. That’s what happens if you don't go light with compounds like 2-Ethyl-3-Methyl Pyrazine. In confectionery, baked goods, snacks, and savory profiles, starting low and building up lets you find the signature taste without veering into artificial territory.
Some people jump at the thought of "more flavor" as an answer, but this doesn’t work for roasted pyrazines. At higher levels, things can turn medicinal or even acrid. I’ve watched junior formulators fall into this trap, overdoing it in search of a bolder nutty or cocoa note, only to receive puzzled looks from panelists who can’t quite pin down what went wrong.
Quantitative guidance helps keep things safe, too. Regulatory agencies like FEMA and EFSA have both cleared 2-Ethyl-3-Methyl Pyrazine for use in foods at what seems like minuscule levels. FEMA GRAS lists it as generally safe when used responsibly. If you glance at published flavor usage references or supplier specs, you’ll see suggestions to stick to below 3 ppm in finished products, and sometimes even below 1 ppm in delicate matrices. These limits reflect not just flavor, but also toxicology reviews.
It pays to keep in mind the base product. A strongly flavored matrix, like a dark roast coffee, welcomes slightly higher concentrations, where a gentle cereal or dairy snack can get away with even less. Even so, exceeding the threshold rarely delivers a better experience. In my own tests, a fraction of a ppm gave a perfectly toasted peanut nuance in a brittle, while doubling it brought out a sharpness nobody enjoyed.
If anyone hopes to experiment, start with the lowest possible increment, ideally around 0.01 ppm, and carefully build up in tiny steps. Rely on sensory panels, because the human nose picks up tiny variations that machines won’t catch. Blending with other compatible flavors helps round out the profile, and sometimes that's the real secret: it’s not about drowning a product in pyrazine, but letting it fit in like a seasoning in a great recipe.
The bottom line, from my own hits and misses, is that recommended usage levels of 2-Ethyl-3-Methyl Pyrazine sit somewhere between 0.01 and 3 ppm, frequently at the lower end for most foods. It’s one of those situations where patience, practice, and a light hand win out every time.
2-Ethyl-3-Methyl Pyrazine plays a big role in flavor chemistry, and anyone who spends time around aromatic molecules knows the importance of careful storage and handling. Spending my early days in a research lab, I remember the punch this compound’s nutty aroma packed even in small quantities. It doesn’t take much to realize that improper storage leads to off-odors, safety issues, and wasted product.
The golden rule for storing this kind of compound: keep it away from heat and moisture. My colleagues and I always tuck bottles in tightly sealed containers, labeling every flask in sight. Damp air, even a little, can mess with purity. Pyrazines absorb water and sometimes take on unwanted reactions, which only leads to extra cost and effort cleaning up the mess. Room temperature storage works, but steer clear of sunlight or any place that heats up during the day. A cool storeroom or one of those trusty chemical cabinets do fine. The more controlled your space, the lower the chance of surprise aromas or loss in quality.
No one in a working lab wants to deal with leaking jars or spilled powder. Experienced hands always pick airtight glass bottles for these kinds of chemicals. Heavy-duty plastics resist interaction, but glass lets you spot any changes right away. We stack the bottles away from acids and bases. Even with a stable material like 2-Ethyl-3-Methyl Pyrazine, cross-contamination creates confusion and waste. One mix-up wiped out hours of my benchwork once, and nobody enjoys tossing out an entire shelf’s worth of flavoring candidates.
Clear labeling becomes second nature after you see how quickly mistakes snowball. Date every container. Include hazard information and chemical names right on the label. In some outfits, color-coded systems help avoid mix-ups during busy stretches. I once spent an afternoon sorting out a set of unmarked bottles—let’s just say that was a mistake no one wanted to repeat. Packing materials matter, too. Insulate glass to prevent breakage on crowded shelves. A dropped bottle spells trouble, and clean-up always takes longer than you think.
Some might find a nutty, earthy smell from this compound pleasant, but nobody wants it to saturate the whole building. Scented air is not just a nuisance. Full-strength aroma can cause headaches, distract coworkers, and raise unnecessary questions from facility managers. Personal experience taught me the value of a proper fume hood. Gone are the lingering odors and complaints. Opening containers beneath ventilation cuts exposure and keeps unexpected visitors at bay.
Early in my career, I never thought twice about emergencies. After hearing about a spill in a neighboring facility, I changed tack. Clean-up kits, eye wash stations, and sheets with emergency steps now sit wherever I handle aromatics. Keeping a material safety data sheet nearby benefits newcomers and veterans alike. With 2-Ethyl-3-Methyl Pyrazine, it rarely ends in fire or chemical burns, but mild skin or eye irritation isn’t unheard of, and nobody should downplay even small risks.
There’s no magic in protecting these chemicals—just steady application of well-practiced routines. By sticking to reliable practices, like airtight storage, careful labeling, and maintaining a clean, ventilated work area, teams can preserve quality and build safer spaces. It doesn’t just make life easier; it sets a standard that strengthens every part of the workflow. If everyone who worked with flavor ingredients paid attention to these steps, workplace hassles would drop fast.
| Names | |
| Preferred IUPAC name | 2-ethyl-3-methylpyrazine |
| Other names |
2-Ethyl-3-methylpyrazine 2-Ethyl-3-methyl-1H-pyrazine 3-Methyl-2-ethylpyrazine |
| Pronunciation | /ˈtuː ˈɛθɪl ˈθriː ˈmɛθɪl paɪˈreɪziːn/ |
| Identifiers | |
| CAS Number | 15707-23-0 |
| Beilstein Reference | 1718732 |
| ChEBI | CHEBI:142245 |
| ChEMBL | CHEMBL1509677 |
| ChemSpider | 112879 |
| DrugBank | DB14037 |
| ECHA InfoCard | 03b849b4-d95d-4958-8af4-4946791b0611 |
| EC Number | 215-940-6 |
| Gmelin Reference | 85989 |
| KEGG | C16338 |
| MeSH | D065448 |
| PubChem CID | 122172 |
| RTECS number | UJ4375000 |
| UNII | T64K4475KN |
| UN number | Not regulated |
| Properties | |
| Chemical formula | C7H10N2 |
| Molar mass | Molar mass: 122.18 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | nutty, roasted, earthy, popcorn |
| Density | 0.978 g/cm3 |
| Solubility in water | Slightly soluble |
| log P | 1.4 |
| Vapor pressure | 0.0194 mmHg at 25°C |
| Acidity (pKa) | 14.47 |
| Basicity (pKb) | 2.7 |
| Magnetic susceptibility (χ) | -56.5·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.507 |
| Viscosity | 1.067 mPa·s (25 °C) |
| Dipole moment | 2.43 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 317.4 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -5.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4070.6 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed, causes serious eye irritation |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P272, P273, P280, P302+P352, P305+P351+P338, P312, P321, P330, P332+P313, P337+P313, P362+P364, P501 |
| NFPA 704 (fire diamond) | 1-2-0-0 |
| Flash point | 61°C |
| Autoignition temperature | 385 °C |
| Lethal dose or concentration | Oral rat LD50: 2070 mg/kg |
| LD50 (median dose) | LD50 (median dose): 460 mg/kg (Rat, oral) |
| NIOSH | UH9265000 |
| PEL (Permissible) | Not established |
| IDLH (Immediate danger) | IDLH not established |
| Related compounds | |
| Related compounds |
Pyrazine 2-Methylpyrazine 3-Methylpyrazine 2,3-Dimethylpyrazine 2-Ethylpyrazine 3-Ethylpyrazine 2,5-Dimethylpyrazine |