People have looked for ways to capture specific natural aromas for decades, even centuries. The pursuit grew stronger in the twentieth century, as food and fragrance industries saw an increased demand for complex, lasting flavors. Scientists digging into the secrets of earthy, green fragrances traced their roots right into the chemical makeup of nature’s flavors. 2-Ethyl-3-Methoxy Pyrazine emerged from this quest, with research stretching back to the 1960s when flavor and aroma chemists isolated smell compounds from green vegetables, bell peppers, and certain wines. These discoveries changed how the world understood flavor profiles, and opened the door for this specific pyrazine to take its place in a growing menu of synthetic and nature-identical flavoring agents.
Everyone who’s worked with flavorings or aromas knows how important impact compounds like 2-Ethyl-3-Methoxy Pyrazine can be. Used in concentrations that seem almost ridiculous — trace parts per billion — this one punches above its weight in giving off that unmistakable green, earthy, even slightly musty note that can make a wine taste layered, help a bell pepper pop, or round out a snack blend. Commercial samples get packed up for evaluation by food technologists, fragrance blenders, and flavorists looking for consistency batch after batch.
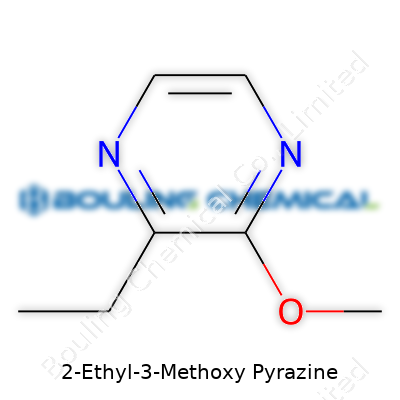
Pour a little sample and the liquid usually runs colorless to pale yellow. It is not particularly viscous, but its scent explodes from the smallest container, overwhelming a workspace with a bell pepper, almost cut grass aroma. Boiling occurs around 148 °C, making it stable enough for many manufacturing settings. It dissolves in alcohol, as many aromatics do, blends with most common flavor solvents, but does not get along with water. Structurally, the chemical formula C7H10N2O explains its low molecular weight and volatility, ensuring it’s noticed by the nose in a hurry.
Product spec sheets from major flavor and fragrance suppliers usually describe purity levels of above 98 percent, analytic confirmation performed by GC-MS, and a specific gravity in the area of about 1.0 at room temperature. Shelf life, provided it’s sealed away from light and oxygen, stretches well over a year. Labeling must show the CAS number 24168-70-5, other regulatory identifiers, and storage recommendations, since small leaks can turn an inventory room into a vegetable patch.
You get 2-Ethyl-3-Methoxy Pyrazine from a series of tried and tested organic reactions. In the lab and industry, folks most often start with 2-methoxy-3-aminopyrazine and hit it with the correct ethylation agent, working under controlled temperatures and pH. Extraction from natural material isn’t practical — the molecule’s concentration in bell peppers and peas clocks in at levels too low for commercial scale-up. Synthetic methods, using high-purity precursors and careful process monitoring, deliver most of what the world needs for food, aroma, and research use.
Curious chemists make tweaks to the pyrazine ring, trying to see how little modifications scramble its scent or crank it up. Reactivity centers around the methoxy and ethyl substituents: swap one out and a new aroma profile pops up. Functionalization can add or block flavors, letting formulators dial in exactly how pungent or subtle they want their product to smell. Oxidation, reduction, or halogenation experiments reveal the stability and possible breakdown byproducts, which helps in testing the safety and stability both in food and perfumery.
Even for those who’ve worked around chemical flavorings, the full list of names might run long: 2-Ethyl-3-Methoxypyrazine, 2-Ethyl-3-Methoxy-1,4-Pyrazine, and methyl-ethyl-methoxypyrazine show up on product lists. In the flavor and fragrance world, sometimes it’s also called “green bell pepper pyrazine.” Such synonyms matter for purchasing, compliance, and cross-referencing flavor libraries across global industries.
Safety takes center stage in any manufacturing or application environment. Even in micro-doses, the compound’s volatility means anyone handling it needs gloves, goggles, and ventilation. Regulatory guidance from agencies like FEMA (Flavor & Extract Manufacturers Association) and the European Food Safety Authority lays out permissible concentrations in food and drink, based on animal studies and broad population exposure. Proper GDP (Good Distribution Practice) guidelines limit contamination or degradation and spell out actions for spills. Storage calls for low light, cool temperatures, and tightly sealed containers labeled for proper hazard communication.
You find this pyrazine across the flavoring spectrum. In wine chemistry, it marks certain varietals and indicates ripeness. In snack foods, it adds depth, echoing roasted or grilled vegetables. Coffee and cocoa blends use a whisper of it to bring out grassy, nutty top notes. Perfumers, especially those after realistic “cut grass” or “wet foliage” accords, rely on its unique green tone. Pet food applications lean on it to mirror the aroma of certain vegetables. Sometimes it even sneaks into trace environmental or malodor research, giving scientists a way to mimic specific off-flavors for training noses.
More research usually means more nuanced use and safer application. Chemists and food technologists keep running sensory panels, analytical studies, and shelf-life tests. Developments in encapsulation allow for precise timed release in processed foods or perfumes. Advances in green chemistry open up possibilities for sustainable synthetic routes, using renewable feedstocks and milder reaction conditions, cutting down on waste and energy demands. Cross-industry teamwork — think food technology, synthetic chemistry, and sensory science — has pushed tools and tricks to keep 2-Ethyl-3-Methoxy Pyrazine both affordable and attractive to buyers.
No one wants a flavoring that causes harm, so toxicology work is crucial. Studies in lab animals tested acute oral, dermal, and inhalation toxicity alongside longer-term studies looking at effects on the liver, kidney, and nervous system. The data so far show that, at use levels common in foods, the risks stay extremely low. Still, regulatory groups regularly review emerging studies on metabolites or breakdown products, and keep their eyes on occupational exposure for workers. Operations managers, QC supervisors, and researchers all stay up to date because safety profiles can evolve with new evidence.
Opportunities stretch in several promising directions. Clean-label and plant-based food trends call for more authentic vegetable notes, opening market space for pyrazines with detailed provenance and safer chemistry. Advances in biotechnology — think engineered yeast or bacteria producing pure flavor compounds — might offer cheaper, eco-friendly sources. Demand from Asia and Africa continues to climb, where cuisines chase not just heat, but also depth and green flavor. If researchers find ways to reduce even trace off-notes or improve stability in complex food matrices, 2-Ethyl-3-Methoxy Pyrazine could show up in even more everyday products, making natural flavors bolder and more memorable while lowering the industry’s carbon footprint.
Step inside a winery, open a bell pepper, stroll past a grapevine—odds are, you’ve encountered 2-Ethyl-3-Methoxy Pyrazine, even if you didn’t know its name. This compound packs a punch with its signature aroma. Fresh, green, snappy and unmistakably reminiscent of bell peppers, it turns out in places you wouldn't always expect. Some days, it lights up the world of wine and food flavor, other days it stirs up both headaches and breakthroughs for quality control experts in agriculture.
Ask any winemaker about pyrazines and you’ll get a grin or a grimace. In some Bordeaux reds, 2-Ethyl-3-Methoxy Pyrazine gives depth and character—a flavor some treasure. Other vintners groan over its tendency to tip over into grassy, unripe territory. For grape folks, it helps tell the story of the harvest. Hotter years zap it out, cooler seasons pull it forward. Getting the right balance isn’t just about finesse, it’s a product of climate, farming techniques, and luck. I remember one vineyard visit where the winemaker shook his head at a batch heavy with this compound. Critics picked up notes of green pepper. Some loved it, some called it a flaw. Either way, it gave the wine personality.
The fun doesn’t stop in the vineyard. Food scientists borrow this molecule for its pop of flavor, especially in products aiming for authentic vegetable notes. Roasted nuts, snacks, even some baked goods lean on it to boost flavor punch. It takes only a drop—literally a trace amount transforms bland into bold. I’ve watched food developers measure this stuff like it’s pure gold. When you see a product that promises “bell pepper flavor,” chances are 2-Ethyl-3-Methoxy Pyrazine had a hand in it.
When pests attack crops like grapes or beans, they sometimes leave behind chemical trails. This molecule gets caught up with pest taint, sabotaging the quality of the final product. For instance, the ‘ladybug taint’ that’s popped up in some North American wines comes from certain beetles crushed with grape harvests—these beetles contain high levels of this same pyrazine. That one small detail can tank months of effort and turn prized produce into a tough sell.
Not everyone wants this compound hanging around. Growers keep an eye out for it because high levels can spoil taste and drop prices. That means constant testing, harvest timing, and pest management. Research teams hunt for ways to tweak grape ripening, filter pyrazines out, or even block their formation in vines. Breeders look for grape varieties less prone to excessive levels. Fine-tuning the vineyard or the lab bench, everyone works to harness the benefits while avoiding the pitfalls.
2-Ethyl-3-Methoxy Pyrazine might sound like chemistry class jargon, but it’s all about how we experience taste and smell. It threads through a surprising part of daily life, showing up in places from your kitchen counter to the world’s best wineries. Anyone serious about food or drink pays attention to this molecule, not out of obligation, but because it’s a flavor gatekeeper. And sometimes, all it takes is one sniff to remind you just how much science shapes every bite.
Open a bag of freshly snipped green bell peppers and a sharp, piercing scent hits the nose. It pokes at memory, often before flavor kicks in. At the core is 2-Ethyl-3-Methoxy Pyrazine, a compound that some know by name, but everyone’s nose knows by experience. I remember walking through the produce section as a kid, bored by the colors, but deeply aware of the green, earthy cloud floating by the bell peppers. Now, digging into food science, I realize that little molecule is the culprit behind that distinct scent.
Anyone who works around this pyrazine knows its punch. The aroma comes across as green bell pepper above all else, but it doesn’t stop there. Many people catch notes of raw green beans, pea pods, and the grassy, sometimes slightly musty air just after mowing a backyard. If you crack open a pea pod, smell fresh cut grass, or split a green jalapeño, there’s a bridge connecting those scents right back to this compound.
2-Ethyl-3-Methoxy Pyrazine can show up in wine, too—think of some Sauvignon Blancs with that notorious “green” character. In the wrong place, it takes a wine from fruity to bitter herb territory. In bell peppers and peas, it feels right at home. It's both a blessing and a curse for winemakers, depending on what kind of profile they aim for.
One aspect that blows people away is just how little of this stuff makes a big impact. Humans can notice it in concentrations as low as parts per trillion. There aren’t many molecules with that kind of sensory clout. In the food industry, this means even trace levels shape the personality of a product. The sensory impact translates into sharp, vivid impressions. If a batch of vegetable soup tastes overwhelmingly “green,” chances are the pyrazines are flexing their muscle behind the scenes.
Growers and winemakers spend years trying to manage pyrazine levels. In cooler climates, grapes hang longer to soften that green streak, but frost and weather play spoiler. I’ve spoken with winemakers in Oregon and France who strategize each season around the risk of under-ripeness, desperate to avoid that bell pepper aroma overwhelming their prized batches.
On the flip side, some culinary traditions lean right into these flavors. Southeast Asian cuisines favor green chilies and fresh herbs, which owe part of their punch to these same molecular notes. Fresh salsas and salads celebrate what others dodge. Context matters, and one cook’s off-note can be another’s signature.
Turning down the volume on 2-Ethyl-3-Methoxy Pyrazine often starts in the field. Canopy management, picking dates, and adjusting sunlight exposure all play a role. Some researchers explore different grape clones less prone to pyrazine buildup. I find it fascinating that such a tiny component can steer so many choices, from vineyard row spacing to chef’s knife.
No molecule tells a bigger story in such a small package. Knowing its impact helps growers, winemakers, chefs, and curious eaters not just chase or avoid it, but better understand how flavor works at the smallest scale.
2-Ethyl-3-Methoxy Pyrazine is a mouthful, but regular folks in food, flavor, and chemical jobs know it by its earthy, green aroma and powerful punch at even low doses. This stuff isn’t something you throw in the back of the cabinet and forget. Over the years, I’ve seen too many good batches go south because a few basic rules got ignored. Temperature, light, and air—these three troublemakers can do a number on pyrazines in general.
Storing this compound at room temperature often works for short periods, but I always aim for the cooler side. Around 4 to 8 degrees Celsius lines up with the typical walk-in fridge in most labs or production spaces. Sure, not everyone has access to a dedicated chemical fridge, though. For folks in smaller outfits, a tightly packed, labeled spot away from heating vents makes a world of difference. Excess heat leads to degradation, and even one hot summer day in the wrong spot can turn a promising batch into an expensive mistake.
Sunlight and even harsh artificial lights chip away at this compound’s quality. I learned early to pick amber glass over clear containers, and to keep bottles in closed cabinets. It seems simple, but a row of clear jars on a sunny shelf looks great while quietly trashing the product. Labels fade, too, and nobody wants to hunt through mystery jars when orders come in.
Oxygen exposure sneaks up as a slow thief, gradually ruining aroma notes and creating off-flavors. Resealable, air-tight lids are basic but crucial. Bigger labs go for nitrogen flushing—purging the bottle headspace before closing things up—to push out oxygen. This habit isn’t just for show; I’ve opened poorly sealed containers more than once and found the sharp green scent all but vanished, replaced by something stale.
Pick containers with tight-fitting seals and no leaky threads. Glass trumps plastic, since some plastics react and mess with the taste. Always scrawl “Open Date” and “Batch” directly on the bottle. Off-the-shelf labeling guns come in handy. If you find yourself digging out old, sticky tape scraps from last year’s batch, it’s time for better storage discipline.
Humidity sits right next to air, heat, and light as a big culprit. I keep desiccant packs around, especially if the lab’s near a cooking area or the weather turns muggy. Business owners jamming everything from flavors to extracts onto the same shelf in a closet often watch entire investments sour. Group like with like, and rotate stock every few months. No excuses for keeping anything past a year if you want reliability.
Knowing and following these steps doesn't only stop waste; it shows respect for everyone’s time and money, from the supplier to the end customer. Ignoring storage turns even the most carefully synthesized batch into a disappointment. If you want consistency in your flavors or research, daily attention to storage pays back every time you open a fresh bottle and smell exactly what you expected.
Open a bag of chips, taste a hint of roasted bell pepper. Try a glass of New Zealand Sauvignon Blanc and pick up that signature aroma. That green, earthy scent often comes from a tiny dose of 2-Ethyl-3-Methoxy Pyrazine (EMP). This compound shapes flavors, especially for foods and drinks chasing that natural, veggie-forward note. Food makers use EMP to punch up taste, and sometimes recreate flavors lost during processing. It’s strong stuff—even tiny amounts go a long way. But as with all food additives, once you hear there’s a chemical name tucked into an ingredient list, people start asking whether it’s safe.
Lab studies show our noses can detect EMP at almost unimaginably low levels. That’s useful for winemakers, who want just a whisper of green; too much EMP can make a glass of wine taste like tinned peas. Researchers have spent decades tracking how EMP behaves, how the body processes it, and what might happen after years of exposure. Most of the safety research comes from animal studies, since scientists can’t feed large doses of added flavor to people for years on end.
According to the Joint FAO/WHO Expert Committee on Food Additives (JECFA) and other evaluators, EMP falls into the group of pyrazines widely used in food flavors across the globe. Regulators allow these because studies haven’t flagged any links to toxicity, allergies, or cancer—at the tiny levels found in food. Anything can become harmful at a high enough dose, even table salt or caffeine. For EMP, the trace amounts used don’t come close to reaching those levels.
I pay close attention to food labels. I watch for flavor enhancers, preservatives, and hard-to-pronounce names popping up in the foods I buy for my family. My concern rises any time an additive becomes trendy. In my kitchen, flavor comes from herbs and slow cooking, but I recognize not everyone has that luxury—processed food helps feed busy lives.
Most people eat or drink compounds like EMP without realizing it. Roasted coffee, peas, bell peppers, and even some beers and wines naturally carry EMP. So do peanuts and dark chocolate. Synthetic EMP matches its natural twin. Flavor chemists rely on these similarities when adding EMP to snacks, soft drinks, or even pet food. Over time, people don’t show ill effects from natural foods containing much more EMP than additives supply.
Regulators don’t give flavorings a free pass. Every permitted compound faces periodic review, especially once new research comes to light. Food safety agencies in the US, EU, and Japan set strict limits for how much EMP can go into a food or drink. Producers must keep levels well below any amount linked with risk.
Given the mountain of data regulators use, and the real-world experience humans get from eating, scientific consensus holds that EMP is safe for its approved uses. But it’s smart to keep asking questions. Demanding transparency in how food is made does more good than harm. The best defense anyone has—learning where food comes from, choosing variety, and keeping both processed flavors and natural ones in balance.
What’s next for chemicals like EMP? Continued research, better labeling, and stricter tracking help. Everyone should have the right to know what’s in every bite or sip. If questions or doubts grow, people can push for even lower use levels or clear-cut, easy-to-read ingredient lists. Until then, pyrazines like EMP stay a tiny piece of a much larger food safety puzzle.
Think about sitting down to a glass of wine or a cup of coffee—one whiff can shape your whole experience. That's often thanks to pyrazines, those tiny molecules with big sensory punch. Among them, 2-Ethyl-3-Methoxy Pyrazine stands out for its potent “green” character: just a whisper conjures up green bell peppers, peas, earth after rain. All it takes is a tiny amount to steer an aroma or flavor in an entirely new direction.
In the world of flavors and fragrances, precision isn’t a luxury; it’s survival. 2-Ethyl-3-Methoxy Pyrazine comes packed with strength—detection thresholds land in the low parts per trillion. A drop too much can turn a blend harsh, grassy, or off-putting. From my years experimenting in kitchen and lab, I’ve seen how novices learn this the hard way: generosity ruins the batch, subtlety brings magic.
Many perfumers and flavor chemists reach for this compound in microgram quantities. It’s not uncommon to work at 0.1 to 2 parts per billion (ppb) in wine, or under 10 ppb in finished food products. I’ve watched flavor teams fuss over microdrops in test vials, knowing that a mere flicker of this intense pyrazine lingers far above its weight. In chocolate or coffee, dishes benefit from vanishingly tiny boosts—often down at 1 to 5 ppb.
Such restraint often baffles newcomers. My early attempts led to bell pepper overload more than once. It’s a learned skill, feeling out the line between subtlety and excess. In some cases, companies dilute the pyrazine in ethanol or propylene glycol to help dose it even more precisely, bringing final content in the finished product down to parts per trillion levels.
Adding too much can turn a beautiful aroma into a failed experiment. Once, I saw a brewery try to amp up the “green” in a beer—customers complained it tasted like canned peas. The lesson stuck. High potency makes for high risk: get heavy-handed and there’s no going back.
One fact stands clear: regulations and safety thresholds establish upper limits for use in food or fragrance, and most practitioners now respect a narrow window—just enough to show the unique edge of pyrazine, not so much as to overpower.
For the flavor development crowd, accuracy comes from testing. Start low. Many blend a trial batch at 0.1 ppb, taste, then inch up by half-steps if needed. Instrumental analysis—gas chromatography with sensitive detectors—helps verify you’re still on target. In practice, most successful products stick to that low-ppb range. Out of hundreds of rounds on R&D panels, those that wow a room almost always land closest to the detection threshold, not far beyond.
For those looking to use 2-Ethyl-3-Methoxy Pyrazine, restraint pays off. Sourcers can buy heavily diluted solutions—for example, 0.01% in alcohol—letting them achieve microgram-level accuracy. Careful record-keeping and batch evaluation stops mistakes from scaling up. If there’s a secret to working with powerful molecules like this, it’s all about balance: smoothness over sharpness, background notes over sledgehammer strength.

| Names | |
| Preferred IUPAC name | 2-ethyl-3-methoxy-1,2-diazine |
| Other names |
Oxyphirazine 2-Ethyl-3-methoxypyrazine 2-Ethyl-3-methoxy-1H-pyrazine |
| Pronunciation | /tuː ˈɛθ.ɪl θriː ˈmɛθ.ək.si paɪˈreɪ.ziːn/ |
| Identifiers | |
| CAS Number | 25676-99-3 |
| Beilstein Reference | 120923 |
| ChEBI | CHEBI:140184 |
| ChEMBL | CHEMBL499758 |
| ChemSpider | 198417 |
| DrugBank | DB14645 |
| ECHA InfoCard | 14d1c10d-60c1-411b-891b-436cdb3fa934 |
| EC Number | 221-136-8 |
| Gmelin Reference | 836934 |
| KEGG | C10590 |
| MeSH | D04754 |
| PubChem CID | 137905 |
| RTECS number | UX8575000 |
| UNII | U0J018A3QD |
| UN number | UN3302 |
| CompTox Dashboard (EPA) | `DTXSID4066262` |
| Properties | |
| Chemical formula | C7H10N2O |
| Molar mass | Molar mass: 138.17 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | Earthy, green, bell pepper |
| Density | 1.05 g/cm3 |
| Solubility in water | Insoluble |
| log P | 1.38 |
| Vapor pressure | 0.0034 mmHg at 25 °C |
| Acidity (pKa) | pKa = 2.23 |
| Basicity (pKb) | “1.56” |
| Magnetic susceptibility (χ) | -69.0 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.501 |
| Viscosity | Liquid |
| Dipole moment | 1.7625 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 221.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -86.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4217.7 kJ/mol |
| Pharmacology | |
| ATC code | |
| Hazards | |
| Main hazards | H302, H317, H319, H411 |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P273, P280, P301+P312, P302+P352, P305+P351+P338, P332+P313, P337+P313, P362+P364 |
| NFPA 704 (fire diamond) | 1-1-0-0 |
| Flash point | Flash point: 93°C |
| Autoignition temperature | 370 °C |
| Lethal dose or concentration | LD50 (oral, rat): 470 mg/kg |
| LD50 (median dose) | LD50 (median dose): >5000 mg/kg (rat, oral) |
| NIOSH | JSS70 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.1 mg/m³ |
| Related compounds | |
| Related compounds |
2-Ethyl-3,5-dimethylpyrazine 2-Isopropyl-3-methoxypyrazine 2-Methoxy-3-isobutylpyrazine 2-Methoxy-3-sec-butylpyrazine 2-Ethylpyrazine 3-Methoxypyrazine |