Chemists have tracked the pyrrole family for well over a century, after the molecule’s discovery in the 1800s. Pyrrole, recognized for its five-membered nitrogen-containing ring, quickly became a platform for all sorts of new chemistry. Out of this class, 2-Cyanopyrrole appeared starting in the late 20th century, drawing attention as more than just a laboratory curiosity. Sharp minds noticed that sticking a cyano group at the 2-position opened a path to tune both electronics and reactivity. Suddenly, 2-Cyanopyrrole shifted from rare catalog item to small molecule that grabbed a spot on many synthetic chemists’ benches—especially as drug design and materials science wanted new scaffolds.
2-Cyanopyrrole stands out in chemical catalogs as an efficient intermediate, offering a direct route into more complex nitrogen-containing compounds. Many researchers recognize its potential for constructing molecules with potential biological activity, since the cyano group provides a handle for attaching more diverse fragments. Industrial producers package this compound as a pale yellow liquid or sometimes crystalline solid, depending on purification and storage. Experienced chemists prefer it as a versatile core, useful for both academic research and process development in industry.
This compound sports a molecular weight around 106 g/mol, falling under the smaller bracket of organic intermediates. Melting points hover near 30 degrees Celsius, often making the compound easier to weigh and handle than more volatile pyrroles. Rooms where solutions of 2-Cyanopyrrole evaporate take on a faint, somewhat sweetish odor, a calling card of pyrrole derivatives that hints at the compound’s aromatic core. The presence of the cyano substituent ensures the compound’s electron density is tuned toward reactivity with electrophiles—a feature that remains valuable for synthetic applications.
Suppliers attach batch numbers and CAS registration (No. 2639-62-9) on every bottle, keeping track for safety and traceability. Bulk users look at purity—most reputable batches clock in from 97% up, and top suppliers share gas chromatography or NMR spectra to support these claims. Labels carry hazard signals reflecting flammability and the need for care when inhaling vapors or allowing skin contact. Packaging usually stays small—often 5- or 25-gram bottles, since research teams rarely get through kilograms before the shelf life could suffer.
Chemists tap into several approaches to access 2-Cyanopyrrole, but the most straightforward route starts from pyrrole itself. Nitrile introduction at the 2-position often relies on a Vilsmeier-Haack or Sandmeyer-type transformation, using reagents like DMF with POCl3 to build a formyl intermediate, followed by dehydration to the cyano group. Careful control at each stage ensures high yields and manages side products, since over-reaction often destroys the sensitive pyrrole ring. Experienced chemists know that handling the intermediates calls for respect due to their own instability, but the method has improved efficiency and safety over the years.
2-Cyanopyrrole plays the role of a flexible starting material thanks to its two reactive features—an electron-deficient cyano group and an aromatic pyrrole ring. The cyano group attracts nucleophilic attack, opening options for hydrolysis to carboxylic acids, reductions to amines, or even cyclization chemistry when reacting with bifunctional reagents. The pyrrole ring itself also handles further substitutions at the 3-, 4-, or 5- positions using standard pyrrole chemistry, giving medicinal chemists a toolkit for small molecule exploration. Many modern drug-development routes include a 2-cyanopyrrole step for precisely this reason: reliable reactivity and robust yields.
Many catalogs list 2-Cyanopyrrole under several names, such as Pyrrole-2-carbonitrile, 2-Pyrrolecarbonitrile, and 1H-Pyrrole-2-carbonitrile. Anyone shuffling through chemical inventories will want to watch for these aliases to keep experiments running smoothly. Some proprietary brand names appear in specialty listings, but scientists determined to minimize confusion stick with the CAS number or systematic nomenclature, keeping ordering and sourcing consistent.
Every lab I’ve worked in treats pyrrole derivatives with a mix of routine and caution. 2-Cyanopyrrole doesn’t pose outsized risks—flammable, irritating on skin contact or inhalation—but it doesn’t allow chemists to drop their guard either. Standard fume hoods become home for transfers and reactions, while gloves and eye protection stay mandatory. SDS (Safety Data Sheets) emphasize moderate acute toxicity through oral or inhalation routes, and disposal follows usual organic waste streams—never straight to drain. Facilities handling multi-kilogram quantities require extra monitoring for spills or accidents due to volatility.
Pharmaceutical researchers look to 2-Cyanopyrrole as a scaffold in the hunt for new therapies, especially for neurological and anti-infective agents. It delivers handy starting material for heterocycle expansion, crucial for targeting biological receptor pockets. Agrochemical developers also benefit, building herbicide and fungicide candidates using this core. Material scientists have pushed the molecule’s derivatives into specialty polymers and liquid crystals, especially where the need for thermal and chemical stability matters. Throughout my own career, I’ve seen it serve as the curiosity that turns into a bread-and-butter tool—always present in the catalog, always a possibility for the next chemical leap.
Research teams continue probing 2-Cyanopyrrole’s potential as a synthetic handle for more complex molecules. As high-throughput screening grows in the pharmaceutical world, need for new building blocks increases—2-Cyanopyrrole lands in many modern reaction screens. Advances in green chemistry have also prompted improvements in how the compound gets made; research groups develop cleaner methods using safer reagents or solvent-saving processes. Many patent applications cite it as a core motif, suggesting that exploration of its reactivity won’t run dry any time soon.
Toxicologists remain cautious with all cyano-substituted aromatics, since both the pyrrole core and the nitrile group have reputations for biological activity. Studies report mild acute toxicity for 2-Cyanopyrrole, especially via oral or inhalation exposure, but chronic toxicity remains less explored in public literature. Researchers test its metabolites closely, worrying about both central nervous system effects and possible carcinogenicity, given the notoriety of some other nitrile compounds. Strict controls in the workplace help, but scientists push for deeper studies using both animal models and computational toxicology to keep surprises at bay as industrial use rises.
The promise of 2-Cyanopyrrole extends beyond current pharmaceutical, agrochemical, and material science applications. As researchers find new catalysts and more sustainable synthetic strategies, this compound could bring reduced environmental impact and better safety profiles. High-value applications such as OLED materials or specialty dyes might rely on derivatives built from the 2-cyano scaffold, pushing into electronics. The fast pace of molecular design and personalized medicine keeps the spotlight on flexible, functional intermediates like this one—it may yet play a role in solving problems that today’s researchers haven’t even identified.
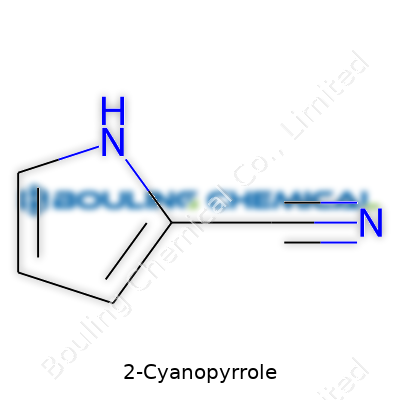
Chemistry doesn’t often make headlines unless something’s gone wrong in a lab or there’s a breakthrough making everyone’s lives easier. 2-Cyanopyrrole falls outside both of those extremes, sitting quietly as a backbone for useful molecules. Its formula is C5H4N2, which means it’s built from five carbons, four hydrogens, and two nitrogens. The name itself adds clues. The “2-cyano” tells you right where the action happens—on the second carbon of the pyrrole ring, you’ve got a cyano group (that’s -C≡N) glued on.
To really see how it fits together, picture pyrrole as a five-membered ring; think of it like a pentagon, but with four carbons and one nitrogen. Stick a triple-bonded carbon-nitrogen cyano group at the carbon next door to the nitrogen atom (the 2-position). Drawn out, you’d have the ring with the cyano group pointing out like a limb. This arrangement shapes how the molecule reacts with other chemicals. The cyano group pulls electrons toward itself, giving the ring different properties than its parent, pyrrole.
Back in my grad student days, I spent a fair bit of time hunched over a bench, chasing new molecules that could change medicine. 2-Cyanopyrrole came up more than once, always as a useful building block on the way to tougher targets. Big pharma likes it too, especially for making certain blood pressure drugs and even antiviral agents. Its structure opens doors for reactions you just can’t get with plain pyrrole.
One of the big draws of adding a cyano group is the flexibility it gives. Cyano groups let chemists tack on all sorts of other chains—think more rings, long carbon tails, or even entire drug scaffolds. In practice, that means researchers can test lots of ideas quickly, tweaking the chemistry until they find something that hits just the right biological target.
Making 2-cyanopyrrole isn’t especially exotic: you start with regular pyrrole, then add the cyano group using reagents like cyanogen bromide. Every organic lab worth its salt has made something similar, though few look forward to handling cyanides or bromides. Both smell awful and pose serious health risks, so safety comes first. I’ve seen entire projects grind to a halt due to mishandling of reactive cyanides. Proper ventilation, gloves, and training matter more here than in most undergraduate experiments.
With green chemistry on everyone’s mind, researchers look for cleaner ways to build molecules like 2-cyanopyrrole. Swapping out harsh reagents in favor of milder, less toxic ones appeals to both ethical and practical concerns. Some groups experiment with electrochemical methods, using electricity instead of hazardous chemicals. Others focus on using recyclable catalysts, trying to leave fewer toxic by-products behind.
On the industrial side, reliable supply chains and better storage methods keep accidents low. Keeping cyanides locked away, sealed from air and water, and only opening bottles in well-equipped fume hoods separates safe chemistry from disaster. Labs use routine training sessions and invest in proper labeling—details that get skipped when rushing, but make the difference between a close call and a catastrophe.
Getting the structure and formula of something like 2-cyanopyrrole right doesn’t just help chemists make novel drugs. It sharpens our sense for which molecules can be built safely and responsibly. That’s the sort of detail that ripples out, from university labs to pharmaceutical companies, changing what ends up on pharmacy shelves or research pipelines. It’s got a small structure but a big role in chemistry’s toolkit.
2-Cyanopyrrole, despite its niche-sounding name, has built a solid reputation inside chemical research labs and pharmaceutical factories. It’s no celebrity, but folks who work in drug discovery or agrochemicals see it around a lot, usually as a stepping-stone for bigger, more complex molecules. Synthetic chemists keep it nearby—mostly because it offers a convenient shortcut to all sorts of nitrogen-based frameworks you'd find in common medicines and crop-protection agents.
In my early days working around process chemists, I saw firsthand how these building blocks help tackle tricky pain points in drug synthesis. Take antiviral research, for example. Teams chase after novel small molecules hoping one might block a virus in just the right way. 2-Cyanopyrrole often serves as a starter, allowing chemists to tack on extra rings, cyanide groups, or saturated side chains. Its structure welcomes transformation, so it fits well into the synthetic toolbox.
A major chunk of medicines—from antipsychotics to antifungals—trace their roots back to pyrrole rings, and the nitrile group attached to 2-Cyanopyrrole opens even more doors for tailoring these rings. APIs (Active Pharmaceutical Ingredients) rarely spring fully formed from scratch. They grow out of smaller, versatile frameworks. 2-Cyanopyrrole lets researchers swap in new pieces at the nitrogen or carbon positions with less fuss than many other building blocks. During my time shadowing project teams, I noticed they’d pick this molecule because it simplified the path toward complicated, patentable structures—saving both time and money.
Folk outside pharmaceuticals might overlook the changes that small tweaks can bring, but something like a nitrile group can change a compound’s life completely. Suddenly, a pesticide gets better soil stability, or a flavor additive lasts an extra season on the shelf. By starting with 2-Cyanopyrrole, agrochemical companies design crop treatments that resist UV breakdown or leach less into groundwater. Crops stay healthier, and fewer chemicals enter waterways. In food science, the same backbone can sneak into artificial flavor pathways, ready to transform into sweeteners or enhancement agents.
Not everything runs smooth, though. The biggest hurdle I’ve witnessed is cost and scale. Small-batch labs whip up 2-Cyanopyrrole easily enough, but scaling production to meet big pharma or agriculture demands gets complicated. That’s partly because the starting materials come from fossil fuels, and folks in the industry keep an eye on carbon emissions and waste. There’s also the matter of purifying the molecule. Any stray impurity can foul up sensitive downstream reactions, making troubleshooting a regular part of the job.
Replacing solvents with greener, less toxic alternatives would help. So would recycling spent catalysts or using renewable feedstocks. Some newer startups experiment with fermentation, using engineered bacteria to build core structures like cyanopyrroles. That approach could shake things up, making bulk chemicals cleaner and cheaper over time.
Folks who rarely set foot in a lab probably won’t recognize 2-Cyanopyrrole, yet it plays a part in keeping modern medicine and crop science moving forward. Instead of chasing after the ideal molecule every time from scratch, chemists lean on flexible intermediates like this one. With the right tweaks, it helps fight stubborn diseases, supports global food security, and may even open new possibilities in eco-friendly manufacturing. Whenever a team lands on a new discovery, there’s a fair chance a humble molecule like 2-Cyanopyrrole kicked off the story.
Working with chemicals like 2-Cyanopyrrole pulls no punches. This stuff doesn’t make the mainstream news, but for folks who spend their time around beakers and bottles, it deserves some respect. Anyone who’s spent a few late nights chasing pure product knows things get messy fast when simple details slip. In my own experience, skipping a step on proper chemical storage caused a mess that I still remember because cleaning up after a preventable spill eats hours. Handling 2-Cyanopyrrole means setting habits for safety from day one instead of waiting until a near miss wakes up the lab.
Let’s get to the point. 2-Cyanopyrrole won’t work out well if it’s left alone in humid, warm, or poorly vented places. Moisture can trigger breakdowns or worse, unexpected reactions. The only smart move is to keep it in tightly sealed containers that don’t let in a drop of water—think high-quality plastic or glass with real gaskets, not whatever is closest at hand.
Temperature drifts matter. Heat builds up indoors, especially in summer, and nitrile gloves only do so much when you’re dealing with a container that’s been sitting near a sunny window. 2-Cyanopyrrole stays happiest between 2 and 8°C, so proper storage fridges earn their keep here. If space runs short, it’s tempting to stash overflow wherever you find room, but leaving reactive chemicals next to the usual solvents close to the fume hood risks all kinds of cross-contamination or worse, accidental mix-ups. Never trust luck with potentially hazardous stock—label everything, and keep detailed inventory sheets that anyone in the lab can decipher without hunting for hidden initials or half-rubbed-off stickers.
In practice, some people take shortcuts out of habit, like setting 2-Cyanopyrrole near strong oxidizers or acids. This shortcut doesn’t save time—it just invites headaches. Mixing up containers, even once, can spark fires or nasty vapors. I watched a close call years ago that went from rowdy laughter to dead silence fast, because someone left open bottles too close together and a routine cleanup came inches from disaster. Store this compound well away from anything that looks like it could react—acids, bases, and oxidizers are all a hard ‘no’ for neighbors.
Gloves and goggles aren’t signs of overkill—they’re just common sense. 2-Cyanopyrrole can irritate skin, eyes, and lungs. I remember the pain of thinking splashed chemicals wouldn’t sting much. The reality: a burning face and a ruined shirt. Always measure small amounts in a well-ventilated hood, never in open air. Clean up spills fast, and use absorbents meant for organic liquids. Never try to wash away a spill with water, since that just spreads things wider and makes tracking the cleanup almost impossible.
There are no shortcuts for accurate records or regular inspections. Relying on a memory or scribbled notes brings mistakes. Set aside time for monthly safety checks, swap out faded labels, and keep a real paper or digital log. If anything goes missing or smells weird, fix it right away—no hand waving or excuses. Better to lose an hour than lose a workday to cleanup or worse.
Good lab habits aren’t about fuss—they build trust and let people focus on what matters most, without worrying the next shift will walk into a crisis. 2-Cyanopyrrole isn’t special in that sense, but inexperience and bad habits make any chemical dangerous. The safest labs run on routines, straightforward records, and a zero-compromise approach to handling tricky compounds.
Anyone who has ever dealt with specialty chemicals knows that purity isn’t just a spec to put in a brochure. It makes the difference between a reaction running smoothly and a batch failing halfway through. 2-Cyanopyrrole, a niche but vital building block in pharmaceutical and agrochemical synthesis, stands as no exception. In the industry, you won’t get far with the stuff unless you’re sure about its purity from the start.
From my own time working with process chemists, the most common purity level that folks expect for 2-Cyanopyrrole lands at roughly 98% or greater. If a supplier says their product hits 99%, they flaunt it. Below 98%, eyebrows go up. This comes from experience, not just books: lower purity material starts causing headaches during downstream steps like N-alkylation or cyclization. It isn’t just about theoretical yields; it’s about avoiding byproducts that chew up time and solvents to clean out.
Suppliers don’t just drop a single purity figure and call it a day. They usually back that up with an assay by GC (Gas Chromatography) or HPLC (High-Performance Liquid Chromatography). On most specs sheets I’ve reviewed, you get something like this:
For projects where regulatory filings get involved, like API routes, quality expectations climb even higher. Tighter control over trace impurities and consistent batch-to-batch assay results become non-negotiable.
The academic crowd may tolerate a bit less, but process scale-up in industry gets expensive fast if you start with material full of dimers, colored tars, or leftover starting pyrrole. Beyond instrument readings, I’ve seen low-quality batches ruin glassware, foul up columns, and slow down every single downstream step. In one project, the sloppiest material added an extra day of work just filtering off byproduct gunk—it wasn’t worth the minor cost saving at all.
High purity also keeps the paperwork light. If a product’s annotated with clean GC traces and tightly controlled specs, no one from QA keeps circling back with questions. It’s easy to track, trace, and troubleshoot rare problems when the baseline product is reliable.
Labs stuck with impure 2-Cyanopyrrole can fight back. Simple distillation on a small scale helps, though yields suffer. Suppliers sometimes try to sneak in “passable” batches when customers don’t push back, so it pays to test new lots yourself with in-house GC. Strong working relationships with known vendors take years to build, but they save a lot of hassle compared to buying on price alone.
To boost the broader supply chain, it helps if users clearly spell out what assay and impurity specs matter for their process and push vendors to provide full chromatograms. At the end of the day, the chemists at the bench need real numbers, not marketing gloss. Pure, well-characterized material keeps projects on track, saves time, and tames costs—something anyone who’s wrangled a batch reaction will respect.
Anybody in specialty chemicals knows how tough it gets sourcing intermediates at scale. 2-Cyanopyrrole is no exception. For a long time, buyers have faced delays, small-batch limitations, or clunky import restrictions. Most chemical manufacturers do not keep hundreds of kilos of this compound sitting on a shelf. Bulk customers—big pharma, flavor and fragrance plants, agrochemical formulators—usually end up negotiating directly for every shipment. Medical chemistry teams, in my experience, adjust lead times to match the unpredictability. It’s rarely just a click-and-ship affair.
Middlemen and distributors often advertise “bulk availability.” In practice, only a handful of certified producers keep capacity open for true high-volume runs. I once saw a team scramble to find consistent 2-Cyanopyrrole for a scale-up. The difference between sourcing a few kilos and a few hundred kilos is night and day. Pharmaceutical-grade material, with certificates, barely emerges on the open market. Commodity-grade material might pop up more often, but buyers end up checking quality twice, sometimes sending samples to third-party labs before signing off on a final order.
Many overlook how important packaging becomes with chemicals like this. Bulk doesn’t always mean drums or barrels. For 2-Cyanopyrrole, packaging choices depend on the volatility, sensitivity to moisture and light, and the planned storage period before use. Suppliers who actually know what they’re doing usually offer steel drums lined inside with protective material—sometimes high-density polyethylene. If the order is big, 200-liter steel drums show up, banded tight, sealed under nitrogen. That slow hiss you hear when unsealing a drum—every lab tech knows the smell.
Those working with less than a drum can expect options like 10 or 25 kilogram HDPE carboys. Anything smaller pushes costs up due to added labor and extra handling. But for larger end users, trying to cut costs on drums often leads to chemical losses or contamination. I’ve seen plenty of spilled product, warped liners, and drums sweating out small leaks—logistics folks remember: proper packing isn’t just a formality. Don’t trust a supplier with poor drum standards. It only takes one slip-up to cost a full batch.
It’s easy to focus on the availability table, but real-world sourcing involves more. Global transport costs, regional regulations on hazardous goods, and shifting demand from adjacent markets all play a role. Last year, as pharma firms scrambled for intermediates, delays stretched weeks out. People who’d built long-term relationships with reliable suppliers pulled ahead. You get what you pay for, and that’s doubly true if you expect fast lead times and consistent purity in bulk.
There’s also the question of sustainability and documentation. Downstream buyers increasingly want to know the source of their chemicals, treatment of waste streams, and regulatory compliance. Document bundles from a legitimate supplier can run a dozen pages per lot, and that paperwork matters more than ever.
You don’t solve the bulk issue with a spreadsheet or just comparing price quotes. The buyers who make things run smoothly spend time building trust with producers and visiting facilities. They demand QA documentation upfront and have backup vendors—even before a crisis. Tech transfer teams know their packaging specs and spell out requirements in advance so that drums or carboys show up exactly as needed. That attention to detail keeps lines moving, and it makes all the difference, especially for fast-paced R&D or tight scale-up timelines. Bulk 2-Cyanopyrrole isn’t impossible to source, but only those with a close eye on logistics, people, and long-term partnerships keep their operations on track.
| Names | |
| Preferred IUPAC name | 1H-pyrrole-2-carbonitrile |
| Other names |
2-Pyrrolecarbonitrile 2-Cyano-1H-pyrrole 2-Pyrrolyl cyanide |
| Pronunciation | /tuː saɪˌænoʊˈpɪr.oʊl/ |
| Identifiers | |
| CAS Number | 20306-43-0 |
| 3D model (JSmol) | `3D model (JSmol)` string for **2-Cyanopyrrole**: ``` CN1C=CC=C1 ``` This is the **SMILES** string for 2-Cyanopyrrole, which can be used as input in JSmol or other molecular viewers to generate the 3D model. |
| Beilstein Reference | 120924 |
| ChEBI | CHEBI:168304 |
| ChEMBL | CHEMBL3179957 |
| ChemSpider | 163858 |
| DrugBank | DB08435 |
| ECHA InfoCard | 100.030.057 |
| EC Number | 872-22-6 |
| Gmelin Reference | 834491 |
| KEGG | C07443 |
| MeSH | D000072633 |
| PubChem CID | 130504 |
| RTECS number | UY7560000 |
| UNII | F4QY8Y86QX |
| UN number | UN3439 |
| Properties | |
| Chemical formula | C5H4N2 |
| Molar mass | 92.10 g/mol |
| Appearance | Colorless to yellow liquid |
| Odor | amine-like |
| Density | 1.08 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 0.03 |
| Vapor pressure | 0.00119 mmHg at 25 °C |
| Acidity (pKa) | 17.0 |
| Basicity (pKb) | 8.15 |
| Magnetic susceptibility (χ) | -46.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.528 |
| Viscosity | 1.37 mPa·s (25 °C) |
| Dipole moment | 2.56 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | S⦵298 = 244.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | 151.3 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1908 kJ·mol⁻¹ |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P273, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362+P364, P501 |
| NFPA 704 (fire diamond) | 2-Cyanopyrrole: 2-2-0 |
| Flash point | Flash point: 102°C |
| Autoignition temperature | 411 °C |
| Explosive limits | Lower: 3.1%, Upper: 14% |
| Lethal dose or concentration | LD50 (Oral, Rat): > 2000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral, rat: 320 mg/kg |
| NIOSH | Not listed |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 2-Cyanopyrrole: Not established |
| REL (Recommended) | 50-500 mg/L |
| Related compounds | |
| Related compounds |
2-Formylpyrrole 2-Aminopyrrole 2-Bromopyrrole 2-Methylpyrrole |