Exploring the roots of 2-Cyano Pyrazine, it’s clear that its story winds through the 20th century’s chemical revolution. Back in the early days of pyrazine chemistry, researchers were on the hunt for nitrogen-containing heterocycles that might serve as useful building blocks or intermediates. The synthesis of cyano derivatives, including 2-Cyano Pyrazine, followed naturally as chemists learned that adding a cyano group could open doors to more reactions and end uses. By the 1960s, as analytical instruments grew sharper and methods cleaner, the structure, reactivity, and potential of 2-Cyano Pyrazine started becoming clearer. Laboratories across the world, from Germany to Japan, began producing and cataloging it, believing its versatility would serve future pharmaceuticals, agrochemicals, and even advanced materials.
2-Cyano Pyrazine belongs to the family of aromatic heterocycles, packing both a six-membered ring with two nitrogens and a highly reactive nitrile moiety. Its backbone isn’t just a dull scaffold; in my experience, it forms a great starting point for a surprising variety of chemical transformations. In catalogues, the compound pops up as a pale yellow solid or sometimes a crystalline powder, with chemical suppliers touting its purity grades for research and industry. Given the regulated and documented handling, much of it moves in standardized containers, complete with batch records and transportation information. Whole careers have developed new molecules from this single core, especially in sectors with a need for nitrogen-rich compounds.
The structure of 2-Cyano Pyrazine, C5H3N3, brings together stability and reactivity. With a molecular weight hovering around 105 g/mol, it melts between 70–74°C, which lets it sit safe on a shelf through a range of ambient temperatures. Its solubility reflects how polar its nitrile group is, so it dissolves well in solvents like ethanol, dimethyl sulfoxide, and even hot water, but it laughs off attempts to blend into nonpolar liquids. Its appearance—pale, almost bland to the eye—masks a sharp, nutty, sometimes bitter aroma, typical of nitrile-containing heterocycles. Chemically speaking, the electron-withdrawing nature of the cyano group at position 2 affects the whole ring, making specific positions ripe for functionalization by nucleophiles or oxidants.
Producers sell 2-Cyano Pyrazine with purity often exceeding 98%, listing impurities like moisture content and any trace byproducts from its synthesis. Labels on commercial containers don’t skimp on compliance, listing CAS number (like 6563-12-8), GHS pictograms, and any relevant storage precautions. Lot numbers tie each batch to a certificate of analysis, which lets chemists trace any issues quickly. From my years handling chemicals from various global suppliers, nothing speeds up troubleshooting like transparent, thorough technical specs—from melting point data to solvent compatibility and shelf-life guidelines.
Making 2-Cyano Pyrazine draws from both classical and modern synthetic chemistry. One popular approach takes pyrazine itself and runs it through cyanation processes using reagents like cyanogen bromide or copper(I) cyanide, typically under controlled heating. Shifts in synthetic methodology over decades have aimed to boost yield, minimize toxic waste, and avoid the use of volatile cyanide sources wherever possible. Some chemists have moved toward catalytic or microwave-assisted syntheses to achieve better selectivity. The hunt for greener, safer routes continues—cost and safety pressures push chemists to adopt scalable, waste-conscious methods.
For a bench chemist, 2-Cyano Pyrazine offers a starting gate to a range of transformations. The cyano group can convert to amidines, amides, or amines by treatment with the right nucleophiles. Reductive hydrogenation brings out primary amines, while acidic or basic hydrolysis builds carboxamides or carboxylic acids. The aromatic ring, activated by the cyano substituent, takes on new groups at specific sites—useful for building libraries of analogs in drug discovery. Borylation, Suzuki couplings, or substitutions with halides all become possible, often with yields and selectivities that outshine pyrazine itself. From my own work, I’ve seen how this molecule’s versatility can make or break a synthetic plan, especially when researchers need nitrogen-rich or polar intermediates.
The chemical structure carries a handful of names in reference works and product catalogs. Blueprints such as Pyrazine-2-carbonitrile, 2-Pyrazinecarbonitrile, and Pyrazinonitrile refer to the same core molecule. European labs sometimes drop into trade language, calling it “2-Cyanopyrazin,” while US suppliers prefer names derived from IUPAC. The CAS number 6563-12-8 rules all, keeping order among synonyms in procurement systems and regulatory filings.
Any chemical with a cyano group deserves careful respect. Handling 2-Cyano Pyrazine means dealing with its mild toxicity, both by inhalation and skin contact. MSDS documents note the need for gloves, goggles, and fume hood ventilation. Cases of eye or respiratory irritation aren’t rare if containment slips, and accidental ingestion or prolonged exposure—though unlikely—raises the risk for headaches or more serious toxicity if it gets metabolized. Disposal follows firm hazardous waste protocols, with no shortcuts allowed. In my experience, keeping solid, up-to-date training in the lab protects researchers and the environment better than any single piece of gear.
Pharmaceutical development leans heavily on compounds like 2-Cyano Pyrazine. Its structure forms the backbone in candidate drugs for epilepsy, antimalarial treatments, and oncology research. Agrichemical companies use it as a starting point for pesticides, seeking out derivatives that show good plant selectivity and environmental breakdown profiles. In the realm of materials science, it plays a role in designing organic semiconductors or specialty dyes, especially where nitrogen content is important for conductivity or color fastness. The flavor and fragrance sector sometimes toys with its use for nutty or roasted notes, though not directly for end-user products due to toxicity.
Academic labs and corporate research groups dig deep into 2-Cyano Pyrazine’s potential. Fellows in medicinal chemistry constantly rework its core, searching for compounds with better biological activity and safer metabolic profiles. Newer research has shifted toward using it in green chemistry, as catalytic protocols and flow synthesis gain traction. Patents turn up with regularity, covering both new analytical methods for detection and whole new synthetic routes. Work in advanced analytics means mapping every possible impurity and tracing even minor reaction side steps—a job that goes hand-in-glove with regulatory requirements. The feedback from these efforts often cycles back, driving production changes and new product launches.
Toxicological studies on 2-Cyano Pyrazine focus on its metabolism and breakdown products, especially any conversion to free cyanide under extreme conditions. Animal models suggest acute toxicity at higher doses, with LD50 values underscoring the need for sensible handling practices. Research in environmental science investigates its breakdown in soil and water, since uncontrolled release could impact ecosystems. Thankfully, under most industry handling scenarios, risk drops sharply when proper ventilation, gloves, and containment are in play. Regulatory reviews in places like the EU and US keep its usage under a close watch.
Interest in 2-Cyano Pyrazine looks set to increase, driven by the quest for new pharmaceuticals and progress in sustainable synthesis. As computational chemistry and AI-guided synthesis become routine, more analogs and derivatives pop up for screening. Synthesizing greener, safer, and higher-yielding routes will shape its industrial future. Safer alternatives may emerge, but the core reactivity and utility offered by 2-Cyano Pyrazine means it stays in the running. My hunch is that the next chapter unfolds in tandem with biotechnology and environmental science, with strict oversight and smarter, cleaner chemistry taking the lead.
2-Cyano pyrazine often flies under the radar compared to headline-grabbing chemicals. In my experience working with scientists and manufacturers, it’s clear: this chemical matters, quietly supporting progress behind the scenes. Its value rests in its versatility and how it enables industries like pharmaceuticals and agriculture to do important work that benefits daily life.
Drug discovery laboratories consider 2-cyano pyrazine a useful starting point. Synthetic chemists favor it because its structure easily adapts to various pharmaceutical candidates. Researchers can tweak its atoms to form different rings and side chains, leading to possible new treatments for infections or disorders that resist conventional therapies. In antimicrobial research, scientists have used it as a template for novel antibiotics. The battle against drug-resistant bacteria presses on, and every scaffold that offers a different mechanism helps broaden therapeutic options. Patents place 2-cyano pyrazine at the root of molecules targeting everything from cancer markers to central nervous system disorders.
Agriculture gets a direct boost from this compound. Chemical companies seeking to safeguard food supply turn to 2-cyano pyrazine as a core ingredient when developing more selective, less environmentally persistent pesticides and herbicides. We’ve seen pressure mount for new ways to fight resistant weeds and harmful insects without polluting water or harming pollinators. When one molecule such as 2-cyano pyrazine leads to safer crop protection agents, that means higher crop yields and fewer residues showing up in the groceries we buy.
Beyond health and farming, researchers treat 2-cyano pyrazine as a stepping-stone for modern materials. It forms the backbone for specialty dyes, which play a role in solar panels, imaging technology, and chemical sensors. Its structure supports efficient electron flow, so when engineers design sensors or displays that need vivid colors and quick response times, components based on pyrazine often find a place in their toolbox. As a result, this chemical resets what’s possible for companies pushing into renewable energy or diagnostic technology.
Anyone involved with 2-cyano pyrazine keeps safety up front. Proper storage, ventilation, and use of gloves and goggles make a difference in avoiding exposure. Regulators watch for documentation on purity and traceability, especially since it serves as an intermediate for substances with health and environmental implications. Trained chemists and technicians rely on established protocols, and companies holding expertise in hazardous materials set the industry standard. Missteps can cause serious problems, so ongoing education and adherence to national guidelines stay vital.
Innovation doesn’t always mean blockbuster discoveries—it can mean refining a single molecule that gives us better medicine, safer crops, and new materials. From my perspective, putting effort into the quiet chemicals, the connectors, brings more sustainable progress than chasing only what’s new and shiny. The next solutions in healthcare or agriculture could stem from a lab where 2-cyano pyrazine makes creative chemistry possible. That’s a future I’d like to see, where science, safety, and everyday benefit meet on common ground.
2-Cyano pyrazine doesn’t sound like something most folks encounter every day outside a lab, but it’s amazing how many chemical names have stories to tell. If you study its structure, you find a six-atom ring with nitrogens at two points, plus a cyano group plugging into the second position of the ring. Chemists break it down through years of tradition: “pyrazine” means that six-ring core, “2-cyano” pins down where the extra nitrogen-carbon triple bond sits.
Let’s break that formula down: Pyrazine itself sits at C4H4N2. The cyano group adds one carbon and one nitrogen, but drops a hydrogen. Add this up—one ring, two extra atoms from the cyano, and a little shuffling of hydrogens—and you get C5H3N3 as the molecular formula. This isn’t some fancy trivia for pub night; you need this data to order reagents, build molecules in the lab, or trace environmental breakdown products.
Back in school, sometimes the formulas for small molecules seemed a bit abstract. But real-life work in chemical safety, medicinal research, and environmental testing drives home why it’s crucial to get things right. The wrong formula means the wrong product—maybe it doesn’t dissolve as expected, or maybe it sets off alarms in a safety system. It’s not just about lab science, either. Manufacturers use these formulas to figure out how much raw material to buy or to blend everything from high-tech coatings to flavor compounds.
People often gloss over details like “N” for nitrogen or “C” for carbon. But confusion between similar chemicals has caused serious problems. Researchers once misread a label and ended up with product recalls that cost millions, all because a molecular formula didn’t match. Quality control teams now double-check molecular weights, and chemical tracking software flags differences between declared and actual ingredient lists.
In drug development, knowing the molecular formula of a compound like 2-cyano pyrazine forms the baseline of trust between research groups, regulatory agencies, and patients. Public databases, like PubChem or ChemSpider, list each entry with its structure and formula for a reason. Cross-checking this formula across suppliers, research articles, and safety data sheets keeps everyone on the same page.
When you visit a production site for food flavorants or pharmaceutical intermediates, traceability requires every drum and barrel to match its paperwork. If the paperwork lists C5H3N3, watchful eyes will check for purity and identity with quick tests—mass spectrometry, infrared, or NMR. These all loop back, in their own way, to that simple molecular formula.
Chemistry doesn’t stand still. Tools like database lookups, barcoding, and automated chemical drawing programs reduce human error. I’ve watched colleagues catch a mislabeled shipment because their system highlighted the wrong number of hydrogens. These solutions make chemical supply chains and laboratory work safer. Emphasizing accuracy with basic data, like the molecular formula for 2-cyano pyrazine, prevents confusion and strengthens the foundation of science-based industries.
Chemicals like 2-cyano pyrazine don’t often make the news. Yet, understanding what they can do to human health matters a great deal for anyone who works with them or lives near sites where they’re stored. My background has brought me into contact with all sorts of industrial laboratories. With that experience, I’ve seen how people can get complacent about “niche” chemicals—until something goes wrong.
2-cyano pyrazine carries some baggage. According to material safety data sheets, it can cause irritation if inhaled, swallowed, or touched. That might not sound dramatic, but irritation here means more than a tickle. Some users report burning sensations, headaches, coughing, or nausea in poorly ventilated spaces. Chronic exposure brings its own worries. Nobody wants to spend years in a workplace, slowly breathing in vapors, and find out their lungs don't work as well as they used to.
In handling this compound, splash goggles and gloves aren’t optional. My old coworkers learned that the hard way—one missed glove and you spend the afternoon flushing your skin. Eyewash stations get used more than people like to admit. Pyrazine derivatives like this one may not top the list of “urgent toxins,” but don’t let that fool you. Problems show up over time, especially in manufacturing or research shops that cut corners.
Let's not forget about what happens when chemicals get loose in the water or soil. Studies tracking nitrogen-based organic compounds have shown they don’t easily break down. 2-cyano pyrazine contains a nitrile group, boosting its stability. In my industrial work, we spent considerable money and time on waste treatment because nobody wants these sorts of molecules lingering in groundwater. Flushing chemicals might seem easier in the moment, but downstream towns feel the impact years later.
For labs and factories, routine safety training pays off. Keeping chemicals sealed, using proper fume hoods, and making sure staff respect PPE seems basic, but enforcement slips if budgets get tight. I’ve seen what happens in facilities with lax rules—burned hands, ER trips, and even permanent injuries. It’s not just about government checklists. Safety becomes a culture people carry home with them. You want coworkers to go home healthy, not dealing with rashes or coughs that never fade.
Better substitution could also help. Green chemistry often gets lip service, but in some cases, alternative compounds offer the same performance without as many hazards. Avoiding 2-cyano pyrazine or using it less can protect both people and the environment, but that takes clear communication between researchers, suppliers, and managers.
Chemicals like 2-cyano pyrazine might live in the shadows of industrial progress, but ignoring their impact is short-sighted. Staying informed, supporting research into safer substitutes, and fostering a culture of care within companies lays the groundwork for long-term health, both for workers and the world outside their windows.
2-Cyano pyrazine doesn’t sit on most people’s radar, but spend a few years in a chemical lab and you learn fast why even lesser-known reagents deserve respect. This compound finds its way into pharmaceutical work and organic synthesis. Its growing use puts a big spotlight on safe storage habits, since mishaps with chemicals ripple out—nobody wants a spill or reaction just because of lazy shelf choices.
Moisture in storage areas spells trouble for many chemicals. With 2-cyano pyrazine, excess water can spark slow hydrolysis or influence purity. In one old lab, we had a storeroom that never shook its musty dampness; labels faded, caps rusted, and some key chemicals just didn’t last. So, I always push for a dry atmosphere—silica gel packets, tightly closed vials, and a little spare effort to keep everything above floor level. Simple steps save months or sometimes the entire batch from degrading. Rooms that rarely reach above 40% relative humidity offer much better peace of mind for people and the products.
Temperature control doesn’t mean shoving everything in a fridge. Most manufacturers advise room temperature for 2-cyano pyrazine, usually sitting between 18–25°C. Temperature swings weaken packaging, especially tubes and caps. Even a high shelf near a sunny window can create mini heat waves that drive subtle decomposition. My go-to rule: find a dark, stable spot away from radiators or vents. Cool, steady conditions lower the odds of by-product formation and don’t stress out the packaging. You notice over the years—chemicals feel fresher, and the lab budget feels less of a squeeze.
Many heterocycles show sensitivity to air or UV. Direct sunlight can break bonds or cause color shifts—early warning that something’s gone wrong. I always stick to amber glass bottles for light-sensitive items and limit oxygen exposure by using septum caps when possible. Labs with open shelves facing windows are a red flag. Fact: A handful of studies—including research published in The Journal of Organic Chemistry—show that trace decomposition can start rapidly when slight UV gets in. That minor tint on your reagent’s glass bottle turns out to be more than marketing; it’s a long-term investment.
I once spent 20 minutes hunting a missing bottle, only to find it hidden behind a much larger flask—always label clearly and keep incompatible chemicals apart. For 2-cyano pyrazine, don’t store near acids, oxidizers, or strong bases. Segregation reduces the chance of accidental mix-ups; accidents involving cyanide derivatives may end badly. Safety sheets recommend Class 3 storage due to mild toxicity, but I add my own rule: only trained staff touch, transfer, or weigh it. A clean working area and sturdy, leak-proof containers make all the difference in emergency prevention.
In a spill, fast action counts. Wear gloves, use absorbent pads, and ventilate well. Waste needs sealing in proper containers, marked for hazardous pickup. Staff should practice response routines at least twice a year. Sometimes one overlooked shelf drags the entire team into a mess that could have been prevented by basic vigilance. Smart planning and honest respect for each chemical’s quirks help everyone stay healthier and safer.
People working in chemical labs don’t care about purity on paper—they care about how it shapes the results they see in beakers and reports. For 2-Cyano Pyrazine, labs usually expect specs of 98% or higher. That number, stamped on a COA, makes all the difference. A few tenths of a percent lower, and your synthesis process stops being predictable. Reactions can take a hit: you get unwanted byproducts, columns gunk up, and product yields drop. Nobody likes lost time, wasted material, or repeat experiments. Chemistry—like baking—relies on ingredients acting the way you expect, every time. Impurities muddy the waters and make that impossible.
Purity used to be a trust-based game. Now, spectroscopic tests do the talking. Most suppliers offer 2-Cyano Pyrazine at 98% or 99%. HPLC and NMR data speak loudly for or against quality. At 98%, you’re looking at material that suits a lot of R&D work. Go up to 99% and above, and you hit the level needed for finished pharmaceuticals or agrochemicals where limits on trace metals or residual solvents can mean the difference between approval and rejection.
I remember a time at the bench when our team switched between two batches—one labeled 98% and the other marked 99.5%. It might seem trivial, but the difference showed up in the purity of the final product and the smoothness of our reaction. Purity isn’t a marketing gimmick; it changes workflows.
Drug makers, electronics engineers, and agrochemical companies want high specs not just for marketing, but for safety and function. Subtle tweaks in impurity profiles can cause toxic side-products or unstable intermediates. No surprise, then, that regulatory bodies publish strict guidelines—not to annoy, but to keep consumers safe. For pharma, impurities above 0.1% invite scrutiny. Even for research use, clean material supports reproducible results, letting scientists build on what they learn instead of troubleshooting contamination.
Not every supplier treats purity the same way. Sometimes, I’ve ordered reagents expecting top-tier material and ended up spending extra hours purifying them in-house. That leads to higher costs, scheduling headaches, and sometimes unsafe exposures. So, buyers need to push suppliers for batch-specific certificates, not blanket specs. Ask for details: does the stated purity include only organic content, or does it exclude moisture and residual solvents? These questions save time and trouble down the line.
In my experience, 2-Cyano Pyrazine with 98–99% purity fits most screening and scale-up work. Scale jumps, regulatory filings, or finished FDA products demand documentation and tighter controls—often 99% and up, plus details on metal traces and solvent residues. Chasing higher purity always makes sense when downstream requirements leave little margin for error.
For teams in labs or production, define your needs before placing orders. If you care about time, safety, and repeatable results, check those specs—don’t just assume what’s in the bottle matches what’s on the label.
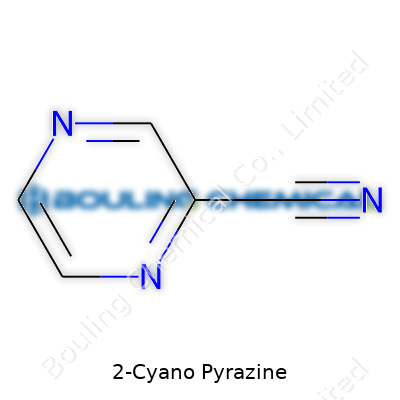
| Names | |
| Preferred IUPAC name | pyrazine-2-carbonitrile |
| Other names |
2-Cyanopyrazine Pyrazine-2-carbonitrile Pyrazinecarbonitrile 2-Pyrazinecarbonitrile |
| Pronunciation | /tuː ˈsaɪənoʊ paɪˈræziːn/ |
| Identifiers | |
| CAS Number | 19772-29-7 |
| Beilstein Reference | 97053 |
| ChEBI | CHEBI:91685 |
| ChEMBL | CHEMBL1491669 |
| ChemSpider | 14261 |
| DrugBank | DB08245 |
| ECHA InfoCard | 03b6e520-7ae8-40dc-8be7-7c11fdf34fae |
| EC Number | 212-515-2 |
| Gmelin Reference | 626793 |
| KEGG | C11684 |
| MeSH | D017901 |
| PubChem CID | 14004 |
| RTECS number | US7875000 |
| UNII | 93R3832J3N |
| UN number | UN3439 |
| Properties | |
| Chemical formula | C5H3N3 |
| Molar mass | 93.09 g/mol |
| Appearance | Light yellow to yellow-green liquid |
| Odor | pyridine-like |
| Density | 1.112 g/cm³ |
| Solubility in water | Soluble |
| log P | 0.08 |
| Vapor pressure | 0.073 mmHg (25°C) |
| Acidity (pKa) | 1.20 |
| Basicity (pKb) | pKb = 9.1 |
| Magnetic susceptibility (χ) | -59.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.499 |
| Viscosity | Viscosity: "1.133 mPa·s (25 °C) |
| Dipole moment | 2.71 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 160.2 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -17.1 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1824 kJ/mol |
| Pharmacology | |
| ATC code | No ATC code |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315: Causes skin irritation. H319: Causes serious eye irritation. H335: May cause respiratory irritation. |
| Precautionary statements | P264, P280, P301+P312, P302+P352, P305+P351+P338, P330, P337+P313, P362+P364, P501 |
| NFPA 704 (fire diamond) | 2-3-0 |
| Flash point | Flash point: 113°C |
| Autoignition temperature | 620°C |
| Lethal dose or concentration | LD50 (oral, rat): 640 mg/kg |
| LD50 (median dose) | LD50 (median dose): 640 mg/kg (oral, rat) |
| NIOSH | Not Established |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 mg/m³ |
| IDLH (Immediate danger) | Unknown |
| Related compounds | |
| Related compounds |
2,3-Dicyanopyrazine 2-Aminopyrazine 2-Hydroxypyrazine 2-Chloropyrazine 3-Cyanopyridine |