Chemists first prepared 2-chlorothiophene in the early twentieth century, not long after foundational work on thiophene itself revealed the utility of sulfur-containing aromatic rings. Early records show German and British researchers focused on halogenated thiophenes to expand the base of functional groups ready for pharmaceutical and agrochemical development. In those days, handling such compounds invited a blend of caution and curiosity, with fume hoods not quite up to today's standards. Over the decades, the demand for new building blocks in synthetic chemistry kept interest in 2-chlorothiophene alive, with each technological leap refining both yield and purity.
2-Chlorothiophene belongs to the thiophene family, with a chlorine atom sitting at the 2-position. In practice, this configuration drives the chemistry of the molecule, affecting where further modification can take place. Anyone working in a research lab can recognize its distinctive, somewhat pungent odor. Commercially available samples tend to be colorless to pale yellow liquids, packed in sealed glass bottles, hinting at its volatility and reactivity. Chemical supply catalogs list several grades, from basic research use to high-purity standards for electronics and pharmaceuticals, priced accordingly.
A closer look at its basic characteristics reveals a boiling point at about 130°C, which means evaporation isn’t a concern until things heat up in the lab. The density sits around 1.25 g/cm³, heavier than water but easy to handle with standard pipetting techniques. Solubility leans toward organic solvents, so you don’t expect much action in water-based systems. The aromatic ring, bolstered by chlorine’s electronegativity and the sulfur atom’s resonance, accounts for both stability and selectivity in reactions. From experience, storing this compound in amber bottles helps preserve quality, especially when exposure to light or air might initiate slow decomposition or chlorination side reactions.
Quality matters, especially for applications in pharmaceutical chemistry, so suppliers rely on GC and NMR analysis to confirm purity levels above 98%. Labels typically display the chemical name, CAS number 1003-09-4, molecular formula (C4H3ClS), and a unique batch code for traceability. Every bottle includes hazard alerts: flammable liquid, irritant, and environmentally hazardous. I’ve often seen QR codes linking to the latest Safety Data Sheet, which proves invaluable for quick reference during audits or training sessions. Lot-specific analytical data, like residual solvent analysis and chlorinated biproduct checks, back every shipment.
Classic synthesis routes begin with thiophene as the starting material and introduce chlorine either through direct chlorination or by substitution reactions. A common approach involves treating thiophene with sulfuryl chloride under controlled temperature to yield 2-chlorothiophene selectively. Careful separation and distillation follow, as the reaction’s byproducts can include 3-chlorothiophene or over-chlorinated compounds. Optimizing temperature, solvent, and chlorinating agent ratio has long been a favorite challenge among process chemists. Recent green chemistry efforts push for milder, less toxic reagents, aiming to avoid the hazards of chlorine gas and to cut down on wasteful byproducts.
The 2-chloro position on the thiophene ring opens the door for nucleophilic substitution, Suzuki or Stille couplings, and other cross-coupling reactions frequently used in big pharma and materials science. Students in advanced organic labs often use palladium catalysts to swap out the chlorine for boronic acids or stannanes, building all kinds of new structures. The electronic effects of the chlorine atom actually guide these reactions, making it possible to design site-specific modifications without much guesswork. Some researchers also look at electrophilic aromatic substitutions, especially on the 5-position, to attach nitro or alkyl groups, expanding the compound’s versatility.
Beyond “2-chlorothiophene”, trade names and synonyms pop up in literature and supply catalogs. “Thiophene, 2-chloro-” and “2-Chloro-1-benzothiole” show up in regulatory filings, while language variations sometimes list it as “chlorothiophen-2” or just “CTP.” Chemical Abstracts uses the standard IUPAC name, but companies may choose their own branding depending on target markets, especially in Asia and Europe. Consistency across labeling, especially for import/export compliance, minimizes confusion. From personal experience, keeping a cross-reference list comes in handy, especially when comparing international specifications or placing multi-source orders.
Proper handling starts with the basics: chemical-resistant gloves, safety glasses, and well-ventilated areas. 2-Chlorothiophene quickly vaporizes at room temperature, so working in a functioning fume hood is non-negotiable. Acute exposure can cause eye and respiratory irritation. If the compound spills, immediate containment minimizes risk to both people and the lab’s environmental control systems. Standard operating procedures demand fire extinguishers and spill kits within reach. Disposal regulations require collection of both liquid and contaminated materials as hazardous chemical waste. Comprehensive team training on these points prevents mistakes and helps keep everyone out of harm’s way.
The main advantage of 2-chlorothiophene comes from its role as an intermediate along the journey to crafting pharmaceuticals and agrochemicals. Medicinal chemists turn to this scaffold to build antifungal, antiviral, or CNS-active compounds, often leveraging the aromatic sulfur’s characteristics for selective binding. Crop science researchers see similar value in using thiophene cores to find novel fungicides and herbicides. Electronics developers explore derivatives in conjugated polymer and organic light-emitting diode (OLED) work, chasing the ideal mix of conductivity and stability. Analytical chemistry relies on its behavior under mass spectrometry for trace analysis protocols. The compound’s adaptability keeps it in steady demand across fields.
Academics and industrial researchers alike chase more sustainable, efficient synthetic routes not just for 2-chlorothiophene but for an entire class of functionalized thiophenes. Recent literature highlights enzyme-catalyzed halogenation and microwave-assisted processes, both sporting lower energy requirements and fewer toxic byproducts. Green solvents and alternative chlorinating agents headline studies out of Europe and Japan, reflecting regulatory pressure to cut down on traditional halogenated waste streams. Open-source databases catalog dozens of unique reactions off the 2-chloro motif, allowing crowdsourced innovation. Young chemists design libraries of derivatives targeting molecular recognition, material science, and environmental sensors, inspired partly by the versatility shown in core thiophene systems.
Reviewing published animal studies suggests low to moderate oral and inhalation toxicity for 2-chlorothiophene, but chronic effects receive less attention. Lab experience shows immediate hazards involve skin and mucous membrane irritation. Environmental toxicity studies raise concerns about persistence in aquatic settings, since halogenated aromatics resist ready biodegradation. Regulatory agencies in Europe call for limits on workplace exposure and careful management of effluent to protect waterways and soil. Routine monitoring and personal exposure records, coupled with advances in predictive toxicology models, support safer handling and smarter policy development. Ongoing work fills data gaps to refine occupational standards.
The future for 2-chlorothiophene ties closely with advances in both synthetic efficiency and application breadth. As pharma pipelines chase molecules with new modes of action, the thiophene ring’s special properties promise to keep this intermediate in heavy rotation. Green chemistry initiatives promise to shrink the environmental cost of production, while automation and in-line monitoring drive up both yield and purity. Outside the pharma world, the demand from flexible electronics, printable solar cells, and new sensors maintains robust interest. From experience collaborating with multidisciplinary teams, the perfect blend—efficient, low-impact synthesis with wide-ranging application—feels within reach, assuming regulatory requirements and market price converge. Each breakthrough in the lab finds its reflection in the supply chain, showing that a classic molecule like 2-chlorothiophene still holds surprises for those ready to push boundaries.
2-Chlorothiophene often pops up in conversations between chemists working in both pharmaceutical and agrochemical fields. Its structure—a five-membered ring containing both sulfur and chlorine—gives it special properties. Rather than being just another lab curiosity, it’s a backbone for building other important molecules. Many pharmaceutical companies source it to develop new drugs, especially those targeting inflammation or bacterial infections. This building block makes complicated molecules easier to synthesize, cutting down the number of steps in large-scale production and making drug costs more manageable over time.
The truth is most people never hear about 2-Chlorothiophene. Yet, it plays a clear role in making common goods safer or more effective. In the world of crop protection, for instance, chemists use it as a starting point for herbicides and pesticides. This matters to anyone who cares about food safety and stable harvests—without these compounds, crops face higher risks from pests and diseases, and that can mean price spikes at the grocery store.
By enabling the production of specialty chemicals, it helps put better-performing products onto the market. Grabbing a painkiller at the pharmacy or picking up fresh produce at the supermarket both involve steps along the chain where substances like 2-Chlorothiophene have played a part, long before the final item lands in your hands.
With benefits come concerns. I remember working on a collaborative project focused on chemical safety. One point kept surfacing: how do you ensure workers and the surrounding environment aren’t harmed during manufacturing or disposal? 2-Chlorothiophene isn’t highly toxic on its own at low levels, but exposure to high concentrations carries health risks. Factories that use it must put strong ventilation and proper disposal systems in place. Chemical leaks or spills, when they happen—especially in places without strong regulations—can affect air and soil quality.
Community members living near chemical plants or research centers need to know what’s going into the air and water. One approach is more transparency. Governments and companies can publish clear data about use, emissions, and incident history, which builds trust and allows people to advocate for better protections. In my view, communities given real-time information about local chemical use respond with more confidence and less anxiety.
Progress never comes from ignoring risks. Tight regulations already shape how companies handle chemicals like 2-Chlorothiophene, but there’s still plenty of room for innovation. Green chemistry aims for smarter syntheses—less waste, fewer hazards, and lower energy use. Chemists today make a point of choosing routes that reduce toxic byproducts or substitute hazardous chemicals for safer ones without sacrificing performance.
To me, the value of 2-Chlorothiophene isn’t just about what it creates but also about how businesses rise to the challenge of using it responsibly. Watching the way new processes unfold shows how health, safety, and product quality all play out over years, not weeks, in ways regular people might not notice at first glance.
In the end, 2-Chlorothiophene shows up where science and daily life meet. From research labs to real-world products, it reminds us that materials science sits quietly beneath the surface of everyday convenience. Keeping one eye on innovation and the other on health and environmental impacts delivers better outcomes all around—something worth pushing for in every industry.
2-Chlorothiophene draws attention for what it represents in the lab and industry. Its molecular formula is C4H3CIS. This breaks down to four carbon atoms, three hydrogen atoms, one chlorine, and one sulfur atom, all arranged in a five-membered ring. The “2-chloro” means the chlorine substitutes a hydrogen at the second position on the thiophene ring. The combo of carbon, hydrogen, sulfur, and a halogen like chlorine isn’t just a trivia answer; this structure shapes its reactivity and makes it valuable for several uses.
Growing up in a blue-collar family, I saw chemistry as a hands-on tool, not just a theory. Understanding a formula like C4H3CIS connects to real work in pharmaceuticals, electronics, and even flavor chemistry. For instance, 2-chlorothiophene fits into production streams for medicines and dyes. Precision matters: getting a molecular formula right prevents multimillion-dollar mistakes in synthesis. Pharma companies lean hard on such details. A single atom makes or breaks a drug’s action or could spark costly regulatory issues. Recent industry audits show that over 30% of quality rejections in synthesis have roots in small but critical structural misassignments.
Digging deeper, the formula points straight to what’s safe and what’s risky. Chlorinated aromatic compounds demand respect. Studies in “Chemosphere” found chlorothiophenes durable enough to stick around in soil and water if handled carelessly. The sulfur atom gives another twist—its oxidation state changes quickly under the right conditions, which causes a chain reaction of byproducts. Chemists who misjudge this let toxic compounds slip through, tainting both surroundings and supply chains.
For tech manufacturers, the presence of both sulfur and chlorine means 2-chlorothiophene helps build electronic components like conductive polymers. Universities working on solar cell research keep the formula top of mind because even a small error in chemical identity can wipe out months of lab work. It’s not about looking smart; it’s about staking jobs, research funding, and even environmental safety on solid knowledge.
Schools teach the theory but often skip the “why.” In the field, knowing that C4H3CIS isn’t just classroom trivia—it’s the fork in the road between safe handling and hazardous mistakes. Sharing detailed molecular info in plain language should be standard practice in workplaces, not hidden in footnotes. As the push for green chemistry ramps up, workers and managers need to see the direct tie between formulas and safe work practices. Regulators and industry leaders could roll out ongoing, scenario-driven training, instead of the usual yearly slide decks, to help embed this knowledge.
Accurate, easy-to-access information bridges the gap between academic chemistry and the floor of a plant or a start-up’s lab bench. In my work with start-ups, I’ve seen organizations turn “unsexy” chemical basics into competitive edges. Figure out the formula, hear out the health and environmental scientists, and odds are good that accidents and product waste drop off. Naming the structure and spelling out why it matters turns industry jargon into a tool everyone can trust.
Dealing with chemicals like 2-Chlorothiophene means walking into an environment loaded with potential risks. This compound’s vapor can irritate the nose and lungs, while liquid forms can sting if they reach your skin or eyes. In my years around labs, even short exposure to similar volatile organics caused irritation that lasted all day. These uncomfortable lessons stick. A few coworkers underestimated fumes, thinking cracking windows did enough. They learned the hard way that smart handling beats shortcuts every time.
Gloves, goggles, and a lab coat come first—always. Nitrile gloves give reliable protection without tearing easily. Splash-resistant goggles shield the eyes from droplets or vapor. A long-sleeved lab coat or chemical apron stops accidental spills from reaching skin or soaking clothes. Closed shoes, not sandals, add another solid layer.
Fresh air makes a noticeable difference with 2-Chlorothiophene. This compound’s vapor isn’t just annoying; it’s a real threat. Work near a fume hood or a well-designed local exhaust system. Relying on “it’ll probably be fine” doesn’t cut it. Some chemists I know used to tape up cardboard to make their own venting — it never worked as well as a true fume hood. Getting headaches after handling volatile chemicals means the setup needs an upgrade.
A safe bottle is a tough, airtight container made for chemicals, clearly labeled, and never stashed near heat or spark sources. Flammable cabinets give extra security. Every time someone gets lazy and leaves a solvent on a windowsill, they forget sunlight cranks up the risk. Proper storage has saved labs from accidents.
Even careful folks face spills. Spill kits help, but only if people know where they are and actually practice using them. Granular absorbents, not just towels, and plenty of ventilation speed cleanup. Thin gloves may seem quick, but chemical-resistant gloves stop burns and lasting damage. Sharing quick stories in trainings, like how someone’s bad spill warped an entire benchtop, gives the warning real weight.
Vapors can overwhelm, especially in busy or small labs. Respirators fit when levels run high or the fume hood’s down. Fit-testing and cartridges designed for organic vapors keep lungs safe. Watching a colleague cough through a poorly chosen mask made me recheck every cartidge type twice before every use.
New lab workers learn fast from others, not just rules tacked to a wall. Peer mentoring, drills, and open talk about close calls turn safety steps into habits. Nobody benefits from glossing over close calls; sharing saves hands, eyes, and futures.
Respect for chemicals like 2-Chlorothiophene grows through sweat, shared mistakes, and steady routines. Every safety measure—gloves, ventilation, smart storage, practiced spill response, protective gear—pays off in real time. Speak up, gear up, and treat every drop with care.
2-Chlorothiophene, recognized by its sharp smell and role in producing medicines, flavors, and agrochemicals, looks simple at first glance. Just a colorless to pale yellow liquid, someone might mistake it for a harmless solvent. Yet, those who spend time in the lab know its hazards can sneak up on the careless. With a boiling point close to 130°C and a flash point hovering around 27°C, this chemical won’t hesitate to release flammable vapor. Those facts alone call for attentiveness from anyone responsible for its storage.
Anyone who has worked with volatile compounds has a story about what happens when storage gets sloppy. Vapors find their way into equipment, sometimes even into the air we breathe. Poor ventilation can ruin more than an experiment; it puts people and property at risk. A locked, well-ventilated flammable cabinet is always worth the investment. It's not just about following regulations—it’s about protecting lives. Insurance policies like to see that setup, but your coworkers appreciate it even more.
Heat and sunlight change chemicals, especially those with sulfur and halogens mixed in. I’ve seen colorless samples turn yellow and start to smell odd after being left near a window. Refrigeration isn’t always necessary, but a cool, shaded, and dry spot makes a difference. Chemical stability isn’t some corporate buzzword. Inconsistent storage temperatures can lead to pressure buildup. Screw-cap bottles sometimes hiss open and vapor escapes, putting the handler at risk for chemical burns or irritation. Safety data sheets spell out compatibility problems, but firsthand experience shows that not every new lab tech reads the fine print.
Water and 2-Chlorothiophene don’t mix well. Storage bottles have to keep out humidity since water can degrade the compound, sometimes creating corrosive byproducts. In the worst scenarios, containers rust from inside, labels peel off, and tracking becomes a headache. Desiccant packs in storage cabinets help, but only if someone remembers to change them. Old hands in the lab keep a sharp eye out for condensation inside bottles—not just because they care about purity, but also because they know that moisture invites disaster.
Glass bottles remain the best option, with PolySeal caps outperforming older styles. Unlike polyethylene, glass doesn’t allow vapor loss or chemical attack, dodging a lot of problems from the start. Manufacturers package this chemical in amber bottles for a reason: the color filters out harmful UV rays, defending against breakdown. Labeling should never take a back seat. Scratched, faded, or missing labels cause confusion and have led to expensive mistakes—even for veteran chemists.
Managing chemicals with real care means more than just shelving them at the right temperature. Regular training keeps everyone sharp. Reviewing storage logs and checking equipment for leaks saves trouble later. Investing in top-quality storage cabinets, enforcing access controls, and cycling stock instead of letting old bottles linger in the back make a difference every day. Accountability and small daily habits build a culture where dangerous accidents become rare. In my own work, the simple habit of sharing stories about close calls did more to educate colleagues than any bland policy memo. Passing that knowledge down means newcomers start with a healthy respect for what goes into every bottle—especially 2-Chlorothiophene.
Labs and factories can’t always afford to see chemicals as mysterious, faceless bottles. Anyone who’s spent time working toward a deadline, or squinted at an obscure chemical safety sheet, knows purity matters deeply. For folks who think 2-chlorothiophene just shows up as a “pure” chemical on the shelf—think again. Lots of chemical suppliers, from the big names in the game to niche specialty shops, bottle this compound in more than one form, and not all grades work for every job.
2-Chlorothiophene helps build drug molecules and flavors—sometimes both in the same decade. In my experience, the question about grades usually comes from someone trying to save money or to nail down exactly how much contamination a process can tolerate. Big pharma research teams usually want a purity grade above 99%, based on gas chromatography. They’re worried that even a sliver of sulfur impurity or a trace of another chlorothiophene could throw off tests, or worse, mess with a key reaction.
For industrial flavors or agrochemical intermediates, some buyers feel fine with a slightly lower grade if it cuts cost and still delivers safe performance. You’ll see grades listed as 97%, 98%, and higher, depending on supplier source and quality control. Not all catalogs make it easy to compare grades, so reading each technical sheet and even talking directly with a rep often becomes part of the routine. Some companies print “synthesis grade” or “analytical grade” on the label, but definitions can change based on region or industry.
I’ve seen mistakes happen when a researcher expects all bottles to be identical. Trace water, heavy metals, or lingering solvents in a supposedly “pure” bottle sometimes pop up as mystery peaks in test data. In one project, a low-grade batch of 2-chlorothiophene brought progress to a halt, forcing a third-party analysis. Turns out, a contaminant at the 2% level triggered an unexpected chemical reaction. These sorts of issues eat into budgets, timelines, and sometimes even safety margins.
Trust between buyer and seller is earned the hard way. Labs work best when chemists can look at a supplier’s certificate of analysis and know it matches what’s in the bottle. Some companies lay out traceability of each lot, and others provide HPLC or GC-MS data upon request. I lean toward the trustworthy suppliers who welcome tough questions about batch history. They want customers to succeed—fewer quality complaints, fewer regulatory headaches.
There’s pressure to cut corners and save costs. Picking the right grade, though, turns out to be a battle between budget and reliability. Running a cheap batch saves some money today, but faulty purities can haunt teams with failed syntheses or recall risks. Teams with experience in these trenches learn to clarify purity needs before placing any order, and they keep detailed notes on supplier performance.
In the end, shopping for 2-chlorothiophene means asking the right questions, reading chemistry data carefully, and keeping suppliers honest. It’s a lesson learned over many years, not a bright slogan in a catalog or a shiny certificate sent by email.
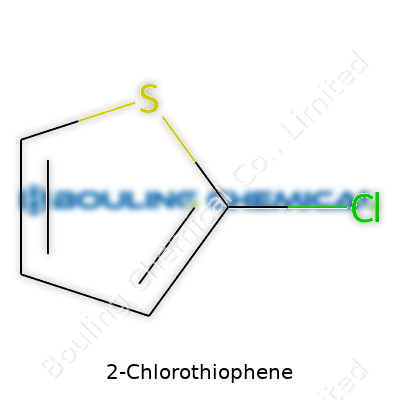
| Names | |
| Preferred IUPAC name | 2-chloro-1-benthiole |
| Other names |
2-Chlorothiophene o-Chlorothiophene Thiophene, 2-chloro- 2-Chlor-1-benzothiophene |
| Pronunciation | /tuː ˈklɔːrəʊˌθaɪ.əˌfiːn/ |
| Identifiers | |
| CAS Number | 96-43-5 |
| Beilstein Reference | 1209222 |
| ChEBI | CHEBI:140365 |
| ChEMBL | CHEMBL37770 |
| ChemSpider | 14115 |
| DrugBank | DB04270 |
| ECHA InfoCard | The ECHA InfoCard of product '2-Chlorothiophene' is: 100.007.886 |
| EC Number | 205-426-2 |
| Gmelin Reference | Gmelin 133736 |
| KEGG | C14125 |
| MeSH | D016697 |
| PubChem CID | 6978 |
| RTECS number | KN4025000 |
| UNII | A1TFH3F9TC |
| UN number | UN2668 |
| Properties | |
| Chemical formula | C4H3ClS |
| Molar mass | 132.62 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | sweet |
| Density | 1.248 g/mL |
| Solubility in water | slightly soluble |
| log P | 2.81 |
| Vapor pressure | 3.5 mmHg (20°C) |
| Acidity (pKa) | pKa = 0.55 |
| Basicity (pKb) | -3.60 |
| Magnetic susceptibility (χ) | -56.5·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.558 |
| Viscosity | 0.613 mPa·s (20 °C) |
| Dipole moment | 1.75 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 165.1 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | 52.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3349 kJ/mol |
| Pharmacology | |
| ATC code | |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H226, H315, H319, H335 |
| Precautionary statements | P210, P280, P305+P351+P338, P337+P313, P403+P235 |
| NFPA 704 (fire diamond) | 2-2-0@string |
| Flash point | 53 °F |
| Autoignition temperature | 490°C |
| Explosive limits | 1.5–11.6% |
| Lethal dose or concentration | LD50 oral rat 1470 mg/kg |
| LD50 (median dose) | LD50 (median dose): >2000 mg/kg (oral, rat) |
| NIOSH | G0375000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 2-Chlorothiophene: Not established |
| REL (Recommended) | 7-10°C |
| IDLH (Immediate danger) | IDLH: 100 ppm |
| Related compounds | |
| Related compounds |
Thiophene 2-Bromothiophene 2-Iodothiophene 2-Fluorothiophene |