The journey of 2-chlorobenzothiazole traces back to the early 20th century, as chemists worked to expand the catalog of heterocyclic compounds. Interest in benzothiazole derivatives really took off with the growth of synthetic dye and pharmaceutical industries. At that time, discovering new compounds wasn’t just about curiosity. It meant unlocking solutions for diseases and opening commercial doors for cutting-edge dyes and pesticides. As research labs started pushing the boundaries of organic chemistry, modifications on the benzothiazole ring led to the introduction of 2-chlorobenzothiazole. This compound didn’t emerge in a vacuum. Industrial demand shaped its development, as the need for more versatile building blocks in chemical synthesis kept growing. Over the decades, the use case for 2-chlorobenzothiazole expanded from niche applications to mainstream usage in research and manufacture, driven by its unique properties and chemical reactivity.
2-Chlorobenzothiazole stands out as an organosulfur compound built from a fused benzene-thiazole ring with a chlorine atom sitting at position two on the thiazole moiety. Its structure delivers an appealing balance between stability and reactivity. Chemists prize such compounds, not just for pure synthesis, but for the way they open up new reaction pathways. In its raw form, the product appears as a crystalline solid or sometimes a pale yellow liquid, depending on the specific grade and storage conditions. This chemical has found its place in the production lines of pharmaceuticals, agrochemicals, and specialty dyes. The demand comes from its ability to serve as a versatile intermediate, meaning it provides a backbone for building more complex molecules.
A good look at 2-chlorobenzothiazole reveals a compound with a melting point ranging roughly between 24-27°C, and a boiling point stretching to nearly 240°C. It doesn’t mix well with water, showing poor solubility, but shows much higher solubility in many organic solvents like chloroform, benzene, and diethyl ether. This chemical’s molecular formula, C7H4ClNS, tells me it combines chlorine, nitrogen, and sulfur in a tightly arranged structure. Its density averages around 1.37 g/cm³, giving it a bit of weight for such a small molecule. If you catch a whiff, expect a pungent, somewhat harsh scent—a reminder of its reactive nature. Stability is fairly reliable under cool, dry storage, yet it reacts vigorously with strong oxidizers. So, storing it thoughtfully becomes non-negotiable.
In commercial trade, purity stands as the key tech specification. Typical batches often guarantee a purity of at least 98%, leaving only a trace of impurities like 2-hydroxybenzothiazole or unreacted benzothiazole. Moisture content controls follow strict limits, as water encourages unwanted hydrolysis. Containers must carry hazard statements under GHS, warning about toxicity and environmental dangers. Labels show not just the chemical name, but alternate synonyms, batch number, manufacturer details, net weight, and proper storage instructions. With its potential to irritate skin and eyes, proper hazard pictograms go on the drum or bottle. Barcode systems streamline warehouse control and traceability, a lesson learned from the demands of pharmaceutical oversight. For safety sheets, regulatory data aligns with the latest standards, including emergency contact information and disposal guidance.
Making 2-chlorobenzothiazole doesn’t call for magic, just precise control and a solid grasp of organic chemistry. Industrial routes usually start with benzothiazole and bring in chlorinating agents like phosphorus pentachloride or thionyl chloride. Temperatures, concentrations, and reaction times find tight control, as even minor deviations can raise impurity profiles or drive down yield. In smaller labs, you can see reactions performed under fume hoods, ensuring chlorine vapors don’t escape into the workspace. Filtration and vacuum distillation round out the process, helping to isolate a clean product free from side products or excess reagents. Over the years, techniques evolved to improve environmental compatibility, favoring methods that minimize halogenated byproducts and reduce waste volumes. Even though green chemistry often struggles with chlorination reactions, ongoing research chips away at these old problems.
On the bench, 2-chlorobenzothiazole serves as a handy electrophile, making it a useful candidate for nucleophilic substitution reactions. Amines step in readily, forming a variety of benzothiazole-2-amine derivatives—many of which possess pharmaceutical potential. Reaction with thiols, phenols, or alkoxides leads to a lineup of possible ethers, thioethers, and substituted products. Cross-coupling reactions open up further possibilities, attaching new aromatic rings for medicinal chemistry or material science. Care during reaction setup pays dividends, since the chlorine atom’s position leaves the ring susceptible to both substitution and elimination, depending on conditions. Each reaction’s choice of solvents and base can skew the outcome dramatically. In hands-on research, unexpected side reactions sometimes yield interesting scaffolds, leading to both useful byproducts and learning opportunities.
It’s easy to overlook the importance of alternate names, but for 2-chlorobenzothiazole, using terms like Benzothiazole, 2-chloro-; 2-Chloro-1,3-benzothiazole; NSC 84453; or even an outdated trade name can create confusion or hazards. In research, database searches must sweep across all known synonyms to avoid missing crucial data or safety information. Patent literature jumps between product names and CAS numbers, adding another layer of complexity. In regulatory filings, companies often stick to IUPAC conventions, yet primary suppliers market it under slightly different commercial-grade descriptions. Being aware of naming conventions ensures smooth logistics, accurate procurement, and sharper compliance.
Handling 2-chlorobenzothiazole calls for real-world caution. Inhalation leads to respiratory discomfort and possible longer-term harm, making fume extraction and proper PPE non-negotiable in professional settings. Safety goggles, gloves, and lab coats form the basic armor for operators. I’ve seen accidents where a careless splash caused dermatitis, so working habits grow out of lessons learned. Spillage control—using absorbents, neutralizing agents, and sealed disposal containers—helps avoid environmental issues and workplace dangers. Safety protocols lean on industry standards set by OSHA and REACH, with risk assessments guiding acceptable exposure limits and handling procedures. Emergency eyewash stations and chemical showers sit within arm’s reach, minimizing risk if things go south. Storage away from oxidizers and in cool, well-ventilated areas cuts down on unexpected reactions. Regular training sessions ensure every staff member respects both the hidden risks and procedural details.
Applications stretch across industrial and research sectors. In pharmaceuticals, chemists rely on 2-chlorobenzothiazole as a precursor for a variety of drug candidates, especially for antimicrobial and anti-inflammatory treatments. Crop protection firms value it for building backbone structures in fungicides and insecticides known for their effectiveness and selectivity. Dyes and pigments draw on its reactivity to introduce bright yellows and deep greens, useful in both textiles and ink industries. Material scientists keep finding ways to blend benzothiazole rings into new polymers and specialty coatings, chasing better performance and durability. Analytical chemists apply it in probe development, tracing contaminants or biomarkers. Every application comes with its own challenges—such as purification needs or application-specific toxicity concerns—requiring creative problem-solving to achieve commercial success without cutting corners on quality or safety.
Innovation means constant experimentation, and 2-chlorobenzothiazole plays a central role in that process. The molecule’s flexible framework encourages the synthesis of novel derivatives. For drug discovery teams, this versatility drives the creation of new lead compounds for everything from antitumor agents to antivirals. Research pads out the academic journals, bespeckled with studies unlocking new reaction conditions or tweaking the molecule for improved performance. Artificial intelligence started playing a role in predicting synthetic routes and biological activity, accelerating trial-and-error cycles. Collaborations between universities and manufacturers aim to trim costs, improve environmental safety, and shrink the carbon footprint of synthetic chemistry. Green chemistry initiatives, including solvent recycling and waste reduction, draw support from industry and academia alike, nudging labs to become more responsible stewards of their chemicals. Patent filings keep growing as new uses—especially in electronics or advanced diagnostics—make headlines.
Toxicological work continues to map the risks of 2-chlorobenzothiazole, as short-term irritation and long-term chromosomal damage become easier to test and quantify. Evidence from in vivo and in vitro studies points to both acute toxicity and possible carcinogenic effects at high exposures, prompting tighter regulation in workplace settings. Animal studies often highlight organ-specific risks, such as liver and kidney damage, if doses climb far above recommended levels. Biodegradation rates and aquatic toxicity remain under scrutiny, since wastewater discharge entered the research radar. Scientists focus on breakdown pathways, aiming to pin down any persistent intermediates that could harm ecosystems. Monitoring and workplace surveillance became regular practice after several high-profile chemical incidents decades ago. Regulatory agencies set strict hazard classifications, while researchers push for alternatives in sensitive applications. Education campaigns and transparency about health effects gained urgency as both academic studies and real-world cases emphasized the need for respect and vigilance.
Looking ahead, expectations for 2-chlorobenzothiazole boil down to three main paths—sustainable synthesis, expanding application areas, and smarter risk management. Trends in pharmaceutical research suggest demand won’t slow anytime soon. Chemical engineers aim to design reactors that cut down on chlorinated waste, meet stricter emission standards, and deliver consistently higher yields. In materials science, researchers seek smart polymers and specialty coatings with fine-tuned electrical or optical properties, pushing the limits of what benzothiazole derivatives can do. Regulatory pressure pushes companies toward greener supply chains, affecting sourcing, manufacturing, shipping, and waste disposal. Digital tracking of every drum or shipment turns from luxury to necessity, as customers and auditors expect end-to-end accountability. Toxicological screening tools keep improving, both in predicting off-target effects and in reducing animal testing. Open data initiatives and international collaboration hope to spread safer practices and faster innovation, as new generations of chemists step up to tackle longstanding weaknesses with fresh ideas and practical solutions.
2-Chlorobenzothiazole carries the formula C7H4ClNS. This compound comes from the benzothiazole family, with a chlorine atom substituting the hydrogen at the number two carbon on the benzene ring. The core structure shows up often in agricultural and pharmaceutical chemistry.
Seeing a formula like C7H4ClNS brings back memories from the college lab, where even a small tweak—like swapping a single atom—changed the outcome of a reaction or how the final product reacted in the real world. 2-Chlorobenzothiazole is no ordinary molecule. The chlorine atom increases electron density and reactivity, making it a valuable anchor point for other chemical modifications.
Industries use this compound in several synthesis routes. For instance, dye manufacturers have relied on it to add stability and colorfastness to pigments. It acts as a starting material for fungicides and pharmaceuticals, sometimes enabling drugs to better reach the site of infection in the body, and giving crops longer shelf life. The role it plays isn’t just about technical chemistry—it’s about end results that show up in people’s daily lives.
Handling 2-Chlorobenzothiazole presents serious safety questions. The chlorine element can trigger reactions which, if mishandled, cause harm to workers or the environment. Studies published in peer-reviewed journals point out that benzothiazoles, as a group, have raised concerns over soil contamination and aquatic toxicity. From what I've seen, waste management protocols make a huge difference. Plant operators need real training and solid risk controls to handle accidental spills or vapors.
Environmental groups flagged benzothiazole derivatives making their way into groundwater. Fact-checking these claims, I came across research that confirmed breakdown products resist natural degradation. Some companies have shifted to closed process systems, which cut down leakage and exposure. Real improvements start with open reporting of spills, regular soil and water testing, and building clean-up plans into production cycles.
Chemical engineers and innovators have looked for alternatives when making dyes or pesticides—less hazardous additives, or ways to cut the chlorine or sulfur loads. Some startups have created filters and absorbents designed to capture benzothiazole compounds from wastewater before it leaves the plant. In a few communities, local watchdogs posted real-time pollution readings online, holding plants accountable and giving neighbors a clear incentive to advocate for cleaner operations.
The story of C7H4ClNS reminds me that every molecule on a bottle’s label tells a bigger story. Each chemical formula points to manufacturing choices, worker safety, and what ends up in the soil and rivers. Science fans and everyday consumers alike benefit from asking what’s inside the materials that touch their lives. Real progress relies on industry transparency and a shared focus on safety, backed by solid science and persistent problem-solving.
Some chemicals seem like they only belong in textbooks or research papers. 2-Chlorobenzothiazole escapes that stereotype. Working a few years in chemical distribution, I learned this compound shows up in more places than most people expect. Producers rely on it for processes that touch pharmacies, farms, and clean water plants.
Farmers wage a constant battle against insects, fungi, and competing weeds. 2-Chlorobenzothiazole becomes a quiet hero in this fight. It’s a key ingredient for synthesizing various agrochemicals, especially those found in pesticides and fungicides. These products help boost yields, reduce waste, and support the production that stocks grocery stores. According to the Food and Agriculture Organization, global crop losses to pests could reach nearly 40% without effective protection. Chemicals based on benzothiazole rings, including this one, play a role in trimming these losses to manageable levels.
Medical researchers often search for new ways to fight infection and chronic illness. 2-Chlorobenzothiazole offers a chemical backbone for several pharmaceutical intermediates. Drug makers use it to build molecules for antibiotics, antifungals, and anti-inflammatory medications. Anyone dealing with a stubborn infection or allergic flare-up might thank the complex web of organic chemistry that includes this compound. The World Health Organization reports rising demand for new drugs as resistance grows, so building blocks like this find demand in medical trials and production facilities.
Public health in cities and small towns depends on safe water. Factories making water treatment chemicals use 2-Chlorobenzothiazole in the synthesis of agents that remove heavy metals and other pollutants. Reliable access to clean water requires a chain of specialty chemistries that start with compounds like this one. Authorities like the Environmental Protection Agency highlight the importance of continuous innovation in water treatment, especially as contaminants shift over time. This isn’t just about industrial water—it extends to drinking water, agriculture, and local ecosystems.
Another surprising area crops up in the color and performance materials we use daily. Manufacturers create specialty dyes for textiles, leather, and paper from 2-Chlorobenzothiazole. The compound’s structure gives stability to colorants, so your jeans stay blue after dozens of washes. The rubber industry, especially for tires and seals, counts on this molecule for vulcanization accelerators. Without these additives, materials wouldn’t last as long on city streets or in machinery. According to the International Rubber Study Group, chemical additives define much of modern rubber’s durability and safety.
The reach of 2-Chlorobenzothiazole brings up tough questions about safety and regulation. Long-term exposure can cause health issues, so chemical plants and processors follow guidelines to limit risks. Training, protective equipment, and environmental monitoring protect workers and communities. For those pushing for sustainability, scientists look for greener synthesis routes and alternative technologies that keep the benefits but limit hazards. Collaboration between industry groups and regulators helps raise the bar for health and environmental standards.
Looking at my own years managing chemical orders, I saw how decisions made at the supplier level echo down to farms, clinics, and city works departments. Reliable chemistry brings better crops, medicine, safe water, and durable goods—but only if handled with care and transparency. By supporting research into new applications and safer practices, we all share the rewards and the risks.
Some folks treat chemical shelf life as just another checkbox. Truth tells a different story. Once, a small lab team I worked with ran into trouble after using an old bottle of 2-Chlorobenzothiazole that’d sat around for years. Funny thing—our results went sideways. Later, we dug into it and realized air and moisture exposure over time had slowly eaten away at the compound’s reliability. Errors like these don’t always shout until a whole batch of effort falls flat.
2-Chlorobenzothiazole is a pale yellow liquid, often carrying a sharp odor. It’s common in organic synthesis, agrochemical research, or even material science labs. In practice, users want consistency and safety above anything else. This only comes when everything stays fresh and undisturbed by what doesn’t belong—like water or heat.
My own rule: treat 2-Chlorobenzothiazole the way you might treat a prized jar of preserves—keep the lid tight, store it cool, and keep sunlight out. Direct sunlight speeds up breakdown. Warm storage and humidity bring real risk of hydrolysis. It’s best to use tightly sealed amber glass bottles and stash them around 2 to 8°C—right about refrigerator temperature but never freezing. That usually works well to stop unwanted reactions and keeps the liquid as close to pure as possible.
If possible, hold stocks in a dry cabinet with silica packs. Humidity can be sneaky—sometimes you think you’re safe, but a foggy day or bad gasket on the storage door does more damage than you imagine. I’ve learned to check the container seal at each use. Many problems start when someone rushes through handling, or worse, leaves the cap off between samples.
Manufacturers label 2-Chlorobenzothiazole for a shelf life between twelve months and two years, but those numbers stand on a foundation of ideal storage. Many folks get by with an extra month or two, but every extra week after the stated expiry chips away at certainty. After about two years, especially if room temperature—or worse, humidity—creeps in, expect breakdown to gather speed. Left too long, degradation products build up and can mess with reactions, spoil purity, or even create minor hazards during lab work.
It doesn’t hurt to check batch records for purchase and opening dates. Opened bottles face more risk. Evaporation, accidental exposure to open air, and condensation gunk up quality without folks noticing until reactivity shifts or unexpected odors crop up. Always date opened containers and keep an eye on color and clarity changes.
Fresh chemicals give peace of mind—and cost less than re-running batches that failed from spoiled stock. I always encourage rotating inventory, buying in smaller batches suited to real usage, and making sure everyone in the lab knows how to handle and store every bottle. Simple actions, like keeping the work area dry and wiping containers before closing, stop little problems before they snowball.
In my experience, chemical safety works best when routine and vigilance walk hand in hand. Storing 2-Chlorobenzothiazole right means you stretch its life, increase safety, and avoid the headaches of unplanned troubleshooting. In short, don’t cut corners—your projects, and your peace of mind, are worth that extra care.
2-Chlorobenzothiazole pops up in conversations about chemical manufacturing, research labs, and wastewater concerns. Just the name sounds complicated, so it’s no surprise folks might worry about possible health risks. Chemicals like this one rarely find their way into the lives of average people, but for those who handle it, understanding the risks can make a big difference between safe handling and potential harm.
This chemical usually turns up in the making of dyes, pesticides, and certain drugs. Some folks in industries working with these products might come across it in raw form. Research shows that 2-Chlorobenzothiazole can escape into the air or water during industrial processes, leading to occupational exposure and environmental release. According to the U.S. Environmental Protection Agency (EPA), improper handling lets small amounts leak out, which might endanger workers or downstream communities.
Spills, leaks, or even just breathing low-level vapors pose real risks for workers. Direct skin contact can trigger rashes or irritation. Breathing in chemical vapors sometimes leads to coughing, shortness of breath, or headaches. Animal testing reveals 2-Chlorobenzothiazole causes toxic effects if swallowed, inhaled, or splashed on skin and eyes. High doses have led to liver and kidney problems in test animals. The World Health Organization(WHO) points out that though no widespread evidence ties this chemical to cancer in humans, long-term exposure brings unknown risks, especially since many chemical-induced diseases take years to develop.
The conversation goes beyond science and labs. It hits home for anyone sharing a workspace or a community near a chemical plant. Work safety depends on consistent training, wearing gloves and safety goggles, using chemical fume hoods, and sticking with local rules for storing and disposing of chemicals. From personal experience, a well-run workplace keeps emergency equipment prepped and everyone knows where to find a Safety Data Sheet for every chemical handled. That sheet isn’t just paperwork; it tells you what symptoms to look out for and when to call a doctor. Too often, folks skip reading it. Slow accidents come from small oversights. Strict safety practice changes that.
No single solution erases the risk of chemical exposure. Still, government agencies and industry groups keep updating rules as new science comes out. The EPA, OSHA, and similar authorities apply limits to air and water releases, set license requirements, and run inspections. Engineers keep working on tighter process control, better filters, and safer substitute chemicals. Community groups have a role too. They organize watchdog programs and demand clear labeling and safe disposal. Technology upgrades alone can’t fix everything—accountability and a safety mindset matter just as much. Demanding transparency and regular monitoring from companies using hazardous chemicals makes neighborhoods safer.
Whether someone works with 2-Chlorobenzothiazole daily or lives near an industrial site, knowing the facts can protect a lot more than just numbers on a safety report. People deserve to recognize when chemicals on-site present a genuine danger, not just another line in a report. The story never stops with paperwork. Careful practice, honest information, and public pressure push decision-makers to take real steps to reduce risk where it starts. Chemical safety is personal, and everyone plays a part in making workplaces and communities healthier.
Walking through chemical supply rooms or scrolling through supplier lists, I’ve noticed that packaging options for compounds like 2-Chlorobenzothiazole shape both how researchers work and how manufacturers make choices. Factories and labs don’t treat this just as a formality—it’s a logistical problem with safety, efficiency, and even budgets riding on the outcome.
2-Chlorobenzothiazole finds its place in different industries, especially in pharmaceutical research and specialty chemicals. Packaging sizes for this compound usually kick off at around 100 grams or 250 grams, scaling upward from small glass bottles to steel drums. The small bottles land with research labs—handy for test reactions and manageable storage. Jars in the 1 kg or 5 kg range often line shelves for pilot-scale experiments or startups, who don’t need truckloads but can’t keep running out after every other synthesis.
For manufacturers or serious industrial users, you’ll see 25 kg fiberboard drums or even large metal containers, tightly sealed to keep moisture out and vapors in check. Flip through some of the big chemical supplier catalogs and you will spot options even up to 200 liters, especially for custom orders. Suppliers sometimes step in with custom sizes if a client’s process calls for it. That’s not a universal promise, but it comes up more often than you’d think.
In labs I’ve worked in, oversized containers just collect dust. They eat up shelf space and increase the risk of spills. Smaller packs, on the other hand, keep waste down, stay fresh longer, and move fast—no one wants to crack open a drum for just a few trial runs. Over in industry, the story flips: opening containers every day means more time spent handling and more chances for exposure, so big drums win out where the volume justifies it.
Glass bottles do the trick for lab use—chemical resistance remains solid and cleanup proves simple. Scale up, and steel or fiberboard containers with tight plastic liners make more practical sense. All this points to one truth: there’s no “right” size in a vacuum. It comes back to safety routines, process timing, and cost—three things every chemist worries about.
The chemical industry has learned the hard way about underestimating packaging. More handling steps open up opportunities for workplace accidents. That’s one reason regulatory agencies, such as the European Chemicals Agency and OSHA, lay out packaging requirements based on risk, not just convenience. Proper labeling, child-resistant closures, and leak-proof drums set a minimum standard. It makes me think about years ago, watching someone spend an hour remixing a split batch because the wrong cap let solvent escape over the weekend. One oversight, and valuable research lost its punch.
Waste raises another problem. Empty drums, plastic liners, and glass bottles add up quickly. Some suppliers encourage users to return drums for cleaning and reuse—a model that cuts down on landfill volume and the costs stuck to single-use packaging. With more companies working toward zero-waste or cradle-to-cradle goals, reusing and recycling shipping containers becomes as important as choosing the right reagent.
Packaging size plays a bigger role in chemical research and industry than many realize. Having the freedom to pick the right format, whether for a university bench or a production line, matters. In my own experience, a well-chosen bottle or drum means fewer headaches, cleaner processes, and safer people. Looking at the big picture, flexible and thoughtful packaging choices are one lever that can pull safety, efficiency, and environmental impact in the right direction.
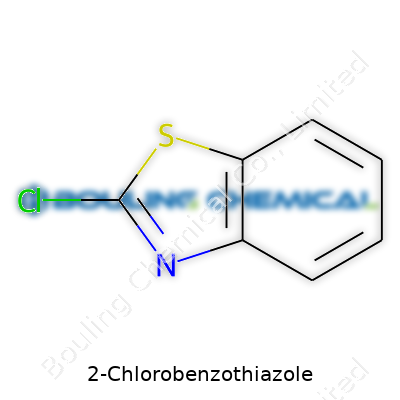
| Names | |
| Preferred IUPAC name | 1-Chloro-1,3-benzothiazole |
| Other names |
2-Chlorobenzo[d]thiazole o-Chlorobenzothiazole Benzothiazole, 2-chloro- |
| Pronunciation | /tuːˌklɔːr.oʊ.bɛnˌzəʊˈθaɪ.əˌzoʊl/ |
| Identifiers | |
| CAS Number | ort: "615-20-3 |
| Beilstein Reference | 120922 |
| ChEBI | CHEBI:38741 |
| ChEMBL | CHEMBL1527 |
| ChemSpider | 11423 |
| DrugBank | DB08242 |
| ECHA InfoCard | DTXSID4020707 |
| EC Number | 213-341-1 |
| Gmelin Reference | 821872 |
| KEGG | C01770 |
| MeSH | D030492 |
| PubChem CID | 6971 |
| RTECS number | DD5250000 |
| UNII | I25503XF91 |
| UN number | UN2668 |
| Properties | |
| Chemical formula | C7H4ClNS |
| Molar mass | 157.63 g/mol |
| Appearance | Light yellow to yellow liquid |
| Odor | aromatic |
| Density | 1.33 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 2.88 |
| Vapor pressure | 0.4 mmHg (at 25 °C) |
| Acidity (pKa) | 2.06 |
| Basicity (pKb) | 11.43 |
| Magnetic susceptibility (χ) | -52 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.6500 |
| Viscosity | 1.28 mPa·s (25 °C) |
| Dipole moment | 3.10 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 222.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | 87.1 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4125 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07, GHS09 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P273, P280, P302+P352, P305+P351+P338, P337+P313, P362+P364, P403+P233, P501 |
| NFPA 704 (fire diamond) | 2-2-0 |
| Flash point | Flash point: 110°C |
| Autoignition temperature | 605°C |
| Lethal dose or concentration | LD50 oral (rat): 670 mg/kg |
| LD50 (median dose) | LD50 (median dose): 670 mg/kg (oral, rat) |
| NIOSH | FM2975000 |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 0.5 mg/m³ |
| Related compounds | |
| Related compounds |
Benzothiazole 2-Bromobenzothiazole 2-Iodobenzothiazole 2-Fluorobenzothiazole 2-Aminobenzothiazole |