Chemists first encountered the thiazole ring back in the 19th century, piecing together its unique structure among a wave of emerging heterocycles. For decades, these sulfur and nitrogen-containing rings sparked questions and possibilities, forming the backbone of dyes and medicines. Later, scientists began focusing on substituted thiazoles, spotting in 2-chloro-5-chloromethylthiazole a valuable handle for building more complex molecules. Its reactivity, especially based on those twin chlorine groups, proved attractive for synthetic and pharmaceutical chemists, as well as those tinkering with crop protection agents. Over time, improved purification techniques and new synthetic methods made the compound accessible with higher purity and at larger scale, responding to the growth of fine chemical industries and the demand for reliable, reproducible intermediates.
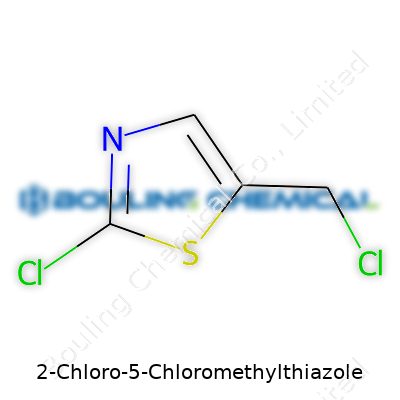
2-Chloro-5-chloromethylthiazole stands out with its two chlorine atoms attached to a five-membered thiazole ring, making it a key intermediate for further derivatization. This compound leaves the lab as a pale yellow crystalline powder or sometimes a viscous liquid, depending on preparation. Both pharmaceutical and agricultural researchers often turn to it as a building block, leaning on its versatility and predictable behavior in a variety of chemical reactions. The product comes labeled with clear warnings and technical information: careful handling, compatibility notes, and, for serious use, certificates of analysis.
Beyond structural formulas and diagrams, working with 2-chloro-5-chloromethylthiazole means knowing how it behaves both in a flask and in the wild. It usually appears yellowish; its melting point and boiling point depend in part on its purity and form, but it hovers in the moderate range common to similar low-molecular compounds. The odor can be pungent—a reminder of the sulfur present in its core. It dissolves best in organic solvents such as chloroform or dichloromethane while showing low solubility in water, which points to careful planning when preparing stock solutions or running reactions. Chemically, the electron-withdrawing chlorine atoms heighten its reactivity, especially at the methyl group next to the thiazole ring.
Manufacturers publish specifications covering purity, moisture content, residual solvents, and identification methods, aiming to meet expectations for both research and scaled-up technical applications. Purity typically sits above 98%, with residue on ignition and volatile impurity content tightly controlled. Labels go beyond hazard icons: expect batch numbers, dates of manufacture, and recommended storage conditions displayed for compliance and traceability. Material safety data sheets, available upon purchase, help responsible users plan their work spaces.
To prepare 2-chloro-5-chloromethylthiazole, chemists usually start with thiazole or a simpler precursor, treating it with chlorinating agents under controlled temperature and atmospheric conditions. Thionyl chloride and phosphorus pentachloride feature in many procedures, with reaction times and work-up techniques tuned to avoid side reactions. Purification often involves distillation or crystallization, and practitioners keep a close eye on reaction progress through techniques like thin-layer chromatography. Production teams working at scale take special precautions to handle byproducts and vent corrosive gases safely, while researchers optimize catalyst usage to cut waste and bump up yields.
This compound’s twin reactive positions—one on the ring, one on the methyl group—open the door for plenty of chemical manipulations. Nucleophilic substitution at the chloromethyl site, especially with amines or thiols, leads to a big family of derivatives. The chlorine atom directly on the ring also participates in coupling reactions or exchanges, which helps chemists bolt on ever more complex fragments. Groups in pharmaceutical discovery often use this core to generate libraries of bioactive candidates, while crop science teams experiment with new fungicides and pest-control agents. The predictable substitution pattern saves time on route scouting and brings down costs.
Chemists won’t always see eye to eye on names, especially across continents or industries. Along with 2-chloro-5-chloromethylthiazole, suppliers sometimes list it under names like 2-chloro-5-(chloromethyl)-thiazole or 5-(chloromethyl)-2-chlorothiazole. CAS numbers, catalog numbers, and sometimes in-house abbreviations fly around in lab notebooks and purchase orders, so double-checking identity before starting a synthesis matters.
Personal experience shows that handling 2-chloro-5-chloromethylthiazole brings familiar chemical hazards—potential to cause burns, eye damage, or respiratory issues if protective equipment falls short. Goggle use, gloves, tight-sealing containers, and work in a properly ventilated fume hood are not optional steps; they’re crucial for daily lab safety. The compound needs cool, dry storage away from both acids and open flames. Formal risk assessments weighing toxicity, volatility, and chemical reactivity guide every experiment or scale-up.
Mixed into the toolbox of modern research, this thiazole derivative supports several industries. Pharmaceutical companies use it to construct drug candidates for infectious diseases and cancer, benefiting from its ability to introduce both chlorine and methyl handles into larger molecules. Agrochemical researchers often use it for insecticide or fungicide development, targeting resistant strains of pests and pathogens with new structures based on the thiazole ring. Material scientists also jump on board, inserting it in specialty polymers or probes for analytical chemistry. Each researcher shapes it to the demands of their own field.
In the push to find new drugs and more efficient crop protectants, research teams study the structure–activity relationships that stem from 2-chloro-5-chloromethylthiazole. Automated systems now screen dozens of derivatives daily, measuring biological activity and environmental persistence. Academic labs tap the compound for synthesis method development, and patent filings show a steady build-up: drug conjugates, imaging agents, specialty resins. Collaboration between industry and university labs ramps up each year, as regulations tighten and the stakes for novel molecular scaffolds rise.
Toxicity sits at the front of every risk evaluation. Tests in rodents highlight acute toxicity at high doses, with organ-specific impacts showing up in liver and kidney function. Inhalation exposure can irritate airways or cause headaches, while skin contact brings redness or allergic reactions over repeated exposure. Researchers study the degradation products, checking whether breakdown yields safer or more persistent compounds. Regulatory bodies look for long-term data on bioaccumulation and chronic exposure, often pushing for substitution or better handling controls if risks appear elevated compared to similar reagents.
Looking ahead, 2-chloro-5-chloromethylthiazole holds promise as a springboard for both greener synthesis and next-generation products. Green chemistry initiatives encourage using milder and less wasteful chlorination methods, aiming to shrink the environmental footprint of large-batch operations. The continued need for smart crop protection tools and new medicinal leads drives ongoing development of novel reactions that start from this core structure. Many hope that tighter operational standards and creative chemistry will unlock safer, more selective derivatives, both lowering risks to users and opening up new commercial pathways. In the hands of trained teams who respect both technical limits and regulatory bounds, this compound keeps its spot as a central building block in the fine chemical world.
2-Chloro-5-chloromethylthiazole comes with the chemical formula C4H3Cl2NS. This molecule tells a bigger story than a line of letters and numbers. Each atom serves a purpose. With four carbons, three hydrogens, two chlorines, a nitrogen, and a sulfur, this structure supports the backbone of many innovations in chemistry.
You’ll find this chemical popping up in places that feed the world. Laboratories use this thiazole derivative as a building block when crafting pesticides. Its presence means improved crop yields and defenses against pests that would wipe out harvests. Growing up around farming, I watched rows of crops fail under pest pressure. A compound like this makes the difference between feast and famine. Responsible use of such molecules keeps food chains moving and prices in check for families everywhere.
It’s easy to overlook the risks that tag along with every bottle of synthetic chemical. 2-Chloro-5-chloromethylthiazole shows toxicity to aquatic life and causes harm with improper handling. Gloves, eyewear, and working in a well-ventilated lab are standard practice for good reason. I’ve seen accidents with thiazole compounds—chemistry shouldn’t take shortcuts. The World Health Organization demands strict storage and waste disposal, and for good reason. Pouring leftovers down the drain brings consequences both environmental and legal.
Every detail in the chemical formula matters. The twin chlorine atoms on the thiazole ring create reactivity. Industry chemists love this, since they can add or switch out other groups on the molecule. That’s how new active ingredients are discovered, improving pesticide performance or lowering human toxicity. I remember long meetings debating the merits of one atomic tweak or another. Each adjustment can bring stronger protection for crops, or a safer residue profile at harvest.
Questions about safety and environmental responsibility follow every new agrochemical. 2-Chloro-5-chloromethylthiazole, with its clear benefits, makes regulators look twice. Scientists in labs run environmental impact studies, and every step faces peer review. Label warnings, dosage limits, and restricted applications show up on packaging, backed by years of data. I’ve watched regulators demand extra testing after unexpected fish die-offs. Their vigilance makes food supply safer for everyone, from growers to dinner tables miles away.
A single formula like C4H3Cl2NS can touch lives in quiet yet powerful ways. This shows how science, regulation, and on-the-ground experience shape safe and useful products. Developing alternatives with lower persistence and toxicity remains a top priority worldwide. Researchers share data, seek out safer byproducts, and work with farmers to minimize runoff. Families deserve safe food. Chemists, farmers, and regulators owe it to each other to keep these standards high, and the story of 2-Chloro-5-chloromethylthiazole serves as a reminder what’s at stake.
Over the years, 2-Chloro-5-Chloromethylthiazole has carved out a valuable reputation for itself in the world of modern agriculture. Many major agrochemical companies rely on this compound to manufacture a range of crop protection products. One of its biggest claims to fame is its use in making thiamethoxam, a well-known neonicotinoid insecticide. By serving as a building block in the synthesis, 2-Chloro-5-Chloromethylthiazole helps bring effective, targeted pest control to millions of hectares of farmland globally. The demand for higher yields pushes innovation, but safety remains critical: proper handling and stewardship in the field keep both food supplies and the environment safer.
The pharmaceutical sector benefits from the versatility of this thiazole derivative. As a core intermediate, it aids chemists in shaping molecules that target very specific biological pathways. Its role in adding complexity and reactivity to molecular structures opens up avenues for new therapies. Some experimental drugs targeting infectious diseases and cancers start with a core structure that this compound helps to build. Formulating new medicines isn’t just about hitting a chemical target—each step, starting from the raw materials, plays into how quickly and affordably healthcare innovation reaches patients.
Laboratories focused on fine chemicals find real utility in this material. Its thiazole ring holds up well during tough reaction steps, which avoids unnecessary waste and keeps projects on course. Custom synthesis projects in dye production, laboratory reagents, and specialty polymers use it as a reliable building block. A scientist on the bench appreciates fewer failed reactions and less cleanup at the end of a long day.
Scaling up a compound like this demands careful management, both in plant design and handling protocols. Manufacturing plants handling chlorinated thiazoles run equipment tested for leaks, since these intermediates aren’t gentle on human tissue or water systems. Without solid engineering controls and emergency procedures, risk multiplies quickly. One memory lingers from an industrial safety training session: missing a simple gasket check turned into a costly shutdown. Even the best chemical becomes a hazard without basic precautions.
In the global market, strict oversight shapes the way 2-Chloro-5-Chloromethylthiazole is bought, sold, and used. Regulatory bodies require data on toxicity, persistence, and exposure risk before granting approvals for downstream products. Farmers, pharmaceutical researchers, and factory engineers depend on clear safety data to make informed choices. Efforts to track and minimize waste, along with regular updates to best practices, encourage a culture of responsibility. Watching local governments strengthen their reporting requirements shows an ongoing shift toward transparency and safer chemical management.
Looking forward, chemical manufacturers and research teams keep working on alternatives and greener solutions. A few projects now focus on solvent-free reactions, reusable catalysts, and routes that cut down hazardous byproducts. It isn't always easy to overhaul older processes inside established factories, but pressure from clients and regulators often forces the issue. Progress may take time, yet every new safer method makes a difference down the line.
2-Chloro-5-Chloromethylthiazole shows up in many labs, often as an intermediate in making pesticides or specialty chemicals. Thiazole rings in general pack plenty of reactivity, and this molecule takes things up a notch thanks to its chloro and chloromethyl groups. A splash or whiff brings real problems: the stuff eats into skin, does a number on your eyes, and its vapors irritate the lungs. Long exposure raises concern for chronic health issues. That points to a chemical demanding extra respect compared to common solvents or mild acids.
I’ve watched folks skip gloves on “quick” transfers and pay dearly. For this one, chemical-resistant gloves make sense. Nitrile or neoprene gloves stand up to halogenated thiazoles much better than latex. Even swapping out gloves between steps cuts down on cross-contamination. Goggles are a must—standard safety glasses don’t shield from vapors or splashes. Faceshields earn their keep, too, when there’s splash risk. Lab coats keep most spills at bay, but long sleeves, closed shoes, and tying back long hair round out the personal protection.
Open windows don’t cut it. Fume hoods give the air sweep needed to nab vapors before they get to you. Even basic handling and weighing improves under a hood. This also zaps traces of vapor before they set off fire alarms or sneak up on people passing by. Portable extraction arms sound nice but lack the capture power of a deep-well hood. And don’t let colleagues store hazardous chemicals outside the vented cabinets—clutter leads to accidents more often than bad luck.
Too many labs tuck these bottles into the nearest shelf, label fading in the sun. Hydrolytic decomposition in contact with water turns some thiazoles into nasty mixtures; humidity makes this worse. Airtight, corrosion-resistant containers work best—straight glass, ideally with PTFE-lined caps. Every container needs a bold, current label, with clear hazard info. I store compounds like this on the lowest shelf behind a spill lip, inside a vented cabinet. That keeps accidental drops from becoming full-blown emergencies.
A written safety plan looks good at inspections, but practice decides who stays safe. Show every team member where the eyewash and shower are, and run practice drills—after seeing a spill once, the muscle memory clicks. Know your emergency contacts, and keep spill kits stocked with proper absorbents (not just old towels). Make sure to have calcium gluconate gel or similar neutralizers on hand in case someone gets skin contact. Double-check that fire extinguishers stand rated for chemical use—water might spread the hazard.
Cuts to corners and complacency can end careers or cause injuries. Data from the U.S. Chemical Safety Board, plus OSHA, show most lab accidents link to basic precaution mistakes. A few bucks on gloves and an extra step for containment prevents a world of hurt, lawsuits, or years off work. People often learn chemical hygiene from coworkers, not textbooks. By sharing solid habits, everyone gets home with vision and skin intact. Even experienced chemists run into problems once; awareness and good habits create safer work for all.
Anyone who has worked around chemicals understands that storage isn’t just about keeping things tidy. It’s about safety, preventing damage, and making sure a chemical like 2-Chloro-5-Chloromethylthiazole doesn’t become a hazard. This thiazole derivative has found use in the chemical and pharmaceutical sectors because it can react easily, but that reactivity means it carries risks. Simple accidents can lead to much bigger problems, so good habits and smart decisions make the biggest difference.
Warm rooms or storage near heat sources do chemicals no favors. The active nature of compounds like this one means they might decompose or become volatile even at slightly higher temperatures. I’ve walked into labs where just a few degrees too warm led to crystallization or breakdown of sensitive reagents. A cool, dry space straight away reduces that risk. Most protocols stick to storing such chemicals below 25°C. No need for freezing, just stable cool conditions away from the window or radiator.
Exposure to light causes certain chemicals to degrade, and there’s no reason to believe this one's any different. Glass bottles—especially amber or brown—do a good job blocking out light, but that’s only half the battle. Humidity can sneak into containers that aren’t shut tight and trigger reactions that make the compound unsafe or useless. Dry air, sealed lids, and silica gel packs go a long way. I've opened jars where one careless moment of leaving the cap off left the contents clumped and useless. Moisture drives trouble in a lab; everyone respects a tight seal for a reason.
Labels get overlooked far too often. Someone working after hours or new on the job may have no idea what’s in that bottle without clear details. The label should show the chemical’s full name, date it arrived, and who opened it. At the same time, odors or dust from compounds like this can irritate airways. Placement in a well-ventilated cabinet cuts this risk. Modern storage cabinets with built-in exhausts keep the working space safer and more comfortable.
Stacking incompatible chemicals on the same shelf spells disaster, and the best labs color-code and separate categories to avoid nasty surprises. Thiazoles don’t play nicely with acids, strong oxidizers, or bases. I learned early that lax storage isn’t just careless; it’s dangerous. Accidental mixing happens quickly, and a splash or whiff can land someone in the ER. Using sturdy, stable shelving and secondary containment pans catches leaks and stops small mistakes from spreading.
Actual safety comes from regular walkthroughs and genuine attention, not from a dusty binder full of policies nobody reads. Routine checks catch leaks, crusty crystal buildup, or unlabeled bottles before anyone gets hurt. It’s the people—lab managers, new hires, everyone—who set the attitude. They build a culture where safe storage is respected and shortcuts don’t get a pass. Modern research, including chemical safety assessments, calls out how accidents almost always follow ignored protocols or casual attitudes instead of actual equipment failures.
Good storage protects people and the future of any lab. Anyone can look up chemical properties, but day-to-day vigilance keeps real risks low. Keep it cool, dry, sealed, labeled, and separated—and accidents start to look a lot less likely.
People hear chemical names like 2-Chloro-5-Chloromethylthiazole and might think it’s just another industrial ingredient. For chemists and makers of pharmaceuticals or crop protection products, it’s another story. Purity isn’t just some number on a sheet — it shapes the outcome, whether in the lab or on the field. Most suppliers deliver this compound at 98% or higher purity. Buyers usually demand a certificate of analysis with tests confirming purity by gas or liquid chromatography. These documents don’t just feel like paperwork; they protect workers, products, end users, and increasingly, the environment.
Specifications for 2-Chloro-5-Chloromethylthiazole center on purity, moisture, and sometimes on residual solvents. For most projects, a purity level above 98% stands as the expectation. The reason is simple: low purity allows impurities to creep into synthesis, leading to unwanted byproducts or poor yields. You find research papers, supplier data sheets, and industry audits reflecting this standard. Unwanted isomers, heavy metals, water content, and organic solvents such as dichloromethane won’t go unnoticed. European and U.S. regulatory agencies treat these contaminants seriously, so checks and documentation grow stricter every year. Missing those guidelines can shut down a batch before it ships.
If you’ve ever worked on a lab project that went sideways without visible reason, purity jumps right up the list of suspects. In my experience, even a single percentage point drop can alter reaction rates or introduce side products. For pharmaceutical intermediates, one impurity can translate into months of remediation or wasted resources. Synthetic routes using chloro-thiazoles sometimes involve strong bases or acids, and leftover impurities might react, creating toxins or reducing safety margins.
The market for thiazole derivatives changes rapidly. A decade ago, few asked what trace byproducts might kidnap a percent or two from yield or purity. Today, everyone from quality control to procurement expects audit trails and chromatography graphs. It’s not rare to see third-party analysis or regulatory spot checks, especially in Europe and North America. If the goal is to ship large volumes for an agricultural product, buyers expect even tighter control, often including specific upper limits for individual impurities — not just a blanket number.
Impurities tend to hide in corners: leftover reactants, traces of elements from glassware, or residual solvents clinging to product grains. One solution comes from better process engineering: tightening up reaction conditions, using high-quality reagents, and flushing equipment between runs. Another approach leans on smart analytics — high-resolution spectroscopy can catch even faint traces. Sometimes, I’ve seen teams benefit just from reworking the workflow: better staff training, stricter record-keeping, and no skipping steps during washing and filtering.
Regulators and customers focus more each year on safety and sustainability. Using products with established purity standards means people down the chain know what they’re getting. Documentation now means more than it used to: it reassures partners, keeps regulators satisfied, and improves yields for everyone.
| Names | |
| Preferred IUPAC name | 5-(Chloromethyl)-2-chloro-1,3-thiazole |
| Other names |
2-Chloro-5-(chloromethyl)thiazole 2-Chloro-5-chloromethyl-1,3-thiazole 2-Chloro-5-chloromethylthiazole 5-(Chloromethyl)-2-chlorothiazole |
| Pronunciation | /tuː-ˈklɔːr.oʊ-faɪv-ˈklɔːr.oʊˌmɛθ.əl-θaɪˈæz.oʊl/ |
| Identifiers | |
| CAS Number | [70258-18-3] |
| 3D model (JSmol) | `/3*;C1=NC(=S)SC1CCl.Cl` |
| Beilstein Reference | 1698734 |
| ChEBI | CHEBI:136733 |
| ChEMBL | CHEMBL415478 |
| ChemSpider | 227132 |
| DrugBank | DB08348 |
| ECHA InfoCard | 18f6ae5a-8ddd-49eb-b6f1-612b7469a6c7 |
| EC Number | 6336-63-8 |
| Gmelin Reference | 906410 |
| KEGG | C14197 |
| MeSH | D031506 |
| PubChem CID | 124045 |
| RTECS number | XN8575000 |
| UNII | 4Y5U5CR865 |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | DJWZTAUZVFYRPU-UHFFFAOYSA-N |
| Properties | |
| Chemical formula | C4H3Cl2NS |
| Molar mass | 181.55 g/mol |
| Appearance | Colorless to light yellow liquid |
| Odor | Characteristic odor |
| Density | 1.52 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 1.98 |
| Vapor pressure | 0.07 hPa (20 °C) |
| Acidity (pKa) | 14.1 |
| Magnetic susceptibility (χ) | -85.0 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.604 |
| Viscosity | 2.23 cP (20°C) |
| Dipole moment | 1.94 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 322.8 J/mol·K |
| Std enthalpy of formation (ΔfH⦵298) | -34.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4731 kJ/mol |
| Pharmacology | |
| ATC code | '' |
| Hazards | |
| GHS labelling | GHS02, GHS05, GHS07 |
| Pictograms | GHS05, GHS07 |
| Signal word | Danger |
| Hazard statements | H301 + H311 + H331: Toxic if swallowed, in contact with skin or if inhaled. |
| Precautionary statements | P210, P261, P264, P271, P280, P301+P312, P304+P340, P305+P351+P338, P332+P313, P337+P313, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) | 2-2-0 |
| Flash point | 68 °C |
| Autoignition temperature | 170°C |
| Lethal dose or concentration | LD₅₀ (oral, rat): 770 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat 240 mg/kg |
| NIOSH | PB2625000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 2-Chloro-5-Chloromethylthiazole is not established. |
| REL (Recommended) | Not established |
| Related compounds | |
| Related compounds |
2-Bromo-5-chloromethylthiazole 2-Chloro-5-bromomethylthiazole 2-Chloro-5-methylthiazole 2-Chloro-5-hydroxymethylthiazole 2-Chloro-5-thiomethylthiazole |