Researchers first documented chlorinated and nitrated imidazoles in the early 20th century. Laboratory discoveries in the mid-1900s drew attention to imidazole rings because they provided the backbone for several essential biological molecules. The introduction of a chlorine atom at position two and a nitro group at position four brought new potency and reactivity into the chemical landscape, sparking innovation in synthetic chemistry. Learning about the early work gives a sense of the long journey it took for chemists to recognize and exploit the substance’s potential, especially as tools improved for manipulating the imidazole core. Laboratories in Europe and the United States set key milestones, using 2-chloro-4-nitro-1H-imidazole not just as an intermediate but as an end-use product when its unique behavior shined in niche applications.
2-Chloro-4-nitro-1H-imidazole appears as a pale yellow crystalline solid, discrete from its simpler imidazole parent and other halogenated analogs. People working in pharmaceutical and agrochemical industries recognize its potential as an intermediate and sometimes as an active ingredient. Its strong electron-withdrawing nitro and chloro substituents put this compound in a reactive sweet spot, a detail not lost on medicinal chemists. Chemical suppliers assign strict batch and purity standards, with certificates of analysis supporting each shipment, and users commonly find it supplied in quantities ranging from grams for research to kilograms for pilot and commercial runs.
2-Chloro-4-nitro-1H-imidazole sits in a molecular weight range around 162 g/mol. It often comes up as a crystalline material, showing limited solubility in water but greater compatibility with polar aprotic solvents like DMF, DMSO, and acetonitrile. Melting points usually fall in the 140–143°C range, signaling robust crystal packing and a fairly stable structure. The chlorine and nitro groups not only change the molecule’s polarity but also affect its electron density, which directly ties into the reactivity seen in substitution or reduction reactions. In my experience, this stability serves product longevity, offering decent shelf life under normal storage. Researchers track hydrolytic stability and photostability, and every fresh container means a new round of tests before the material goes into a valuable synthesis.
Bottles and drums of 2-chloro-4-nitro-1H-imidazole bear hazard labeling consistent with GHS standards, warning about toxicity, irritancy, and environmental risk when disposed of improperly. Typical products register a purity level upwards of 98 percent, and analytical techniques like HPLC and NMR prove batch consistency. Regulatory information often appears right on the label or accompanying safety data sheets, including strict details on material identification numbers, signal words, and handling guidance. Labels reflect trace impurity levels, water content, and batch certification. Laboratories caring about reproducibility avoid using old or improperly stored stock, and audit trails logged by manufacturers support robust traceability.
Most current synthetic pathways for 2-chloro-4-nitro-1H-imidazole proceed by direct nitration and chlorination of the imidazole scaffold under carefully controlled temperature and pressure. Chemists working in kilogram batches rely on an initial protection or activation step based on the desired regioselectivity. Monitored reaction times and purification by recrystallization or chromatography make the difference between a usable product and a failed batch. Waste streams require careful neutralization before disposal—these methods demand tight process controls to avoid byproduct formation that complicates purification or affects subsequent chemistry. Companies patent novel catalytic methods and flow chemistry advances to improve overall yield and selectivity, all to deliver batches with tighter impurity profiles for regulated uses.
2-Chloro-4-nitro-1H-imidazole emerges as a versatile substrate for nucleophilic aromatic substitution, particularly at the C2 position, where the chlorine atom leaves under mild conditions. Its nitro group, meanwhile, offers further scope for reduction, giving amines or hydroxylamines, crucial transformation points for building more complex molecules. Over the years, practitioners have deployed it to craft antifungal, antibacterial, and anticancer scaffolds, seizing on its amenability to Suzuki or Buchwald coupling reactions and introducing further diversity. Opposite to classic halogenated aromatics, the imidazole ring pushes electron density in just the right way to favor specific transformations, and people care about side reactions, so they design conditions to keep yields high. Researchers push beyond single-step transformations, building libraries for screening in pharmaceutical pipelines.
Basketed under a handful of commercial and systematic titles, this compound appears in catalogs as 2-chloro-4-nitroimidazole, 2-chloro-4-nitro-1H-imidazole, and sometimes as NSC number identifiers. Trade literature and lab documentation both stress the specific substitution pattern; careless switching among close relatives means mixed results and lost resources. The use of distinct catalog codes at major suppliers smooths ordering and regulatory tracking when multinational shipments or regulatory filings come into play. Nomenclature differences, especially around tautomeric states or salt forms, confuse less-experienced users, so it pays to verify structure and CAS registration before launching an experiment or process.
Anybody working with 2-chloro-4-nitro-1H-imidazole gets direct experience with its irritant properties—accidental contact brings skin and eye discomfort, while inhalation exposure in the workplace gets flagged by occupational health teams. Labs keep engineered ventilation, gloves, and eye protection in constant supply, and incident logs highlight why attention to containment matters. Storage away from heat, sunlight, and sources of sparks retains product integrity, and segregated waste streams prevent cross-contamination or accidental reactions. Training drills reinforce emergency procedures, and all hands know to consult the safety data sheet before commencing large-scale reactions or scale-up work.
People in the drug discovery and crop protection segments value this molecule as a stepping stone to functional leads and formulation auxiliaries. Medicinal chemists have explored modified imidazoles for their enzyme-binding characteristics, seeing a place for halo- and nitro-imidazoles in antimicrobial and anti-parasitic products. Outside the healthcare arena, these compounds have found a spot in specialty dyes and polymer modification studies, where resilience and targeted reactivity carry benefits not found in older, less functionalized rings. Development chemists mix this compound into pilot programs, weighing cost versus synthetic accessibility with every campaign.
Teams worldwide drive advances in modification strategies, greener syntheses, and exploratory biological screens. Parallel synthesis studies produce small molecules with subtle tweaks to substitution, hoping for increased selectivity or lower toxicity. Results often move from bench scale into computational modeling, generating predictive insights for the next batch of analogs. Academic collaborations bring fresh perspectives, weaving in machine learning to optimize structure-activity relationships, and journals regularly report on the property trends that map back to minor changes in molecular structure.
Investigators have run extensive screens on the toxicity of 2-chloro-4-nitro-1H-imidazole using cell models and animal studies to identify acute and chronic effects. Reports indicate irritation potential and highlight the importance of limiting direct exposure. Inhalation brings respiratory discomfort, and ingestion triggers gastrointestinal symptoms, all of which guide workplace controls. Comparative toxicology literature weighs it against related nitroaromatics, noting that reactivity with biological macromolecules underlies hazard classification. Environmental groups watch discharge limits, and regulatory bodies demand transparent lifecycle analysis before extending new uses outside R&D.
Rising interest in precision Active Pharmaceutical Ingredient (API) synthesis and targeted crop-protection compounds promises a busy future for 2-chloro-4-nitro-1H-imidazole. Researchers target higher-throughput, less polluting synthesis routes, aiming for sustainability benchmarks to meet next-generation compliance. Automation and process analytics streamline production, increasing reliability and squeezing waste out of the workflow. Regulators look for tighter purity and compliance documentation, opening the door for digital tracking and blockchain certification to map every gram of material. Scientists scan the horizon for new biological uses—antimicrobial resistance challenges and emerging therapeutic targets shape each new proposal and patent. The real key to its future rests on the ability to responsibly balance efficiency, safety, and environmental stewardship, building on the decades of experimentation and adaptation that have brought the molecule this far.
2-Chloro-4-Nitro-1H-Imidazole stands out in organic chemistry because of its unique pattern of substitution on an imidazole ring. The name tells you exactly where to look: a chlorine atom at the second position, a nitro group at the fourth, and the imidazole core as the backbone. In my days teaching undergraduate chemistry courses, students often found heterocyclic compounds puzzling until they visualized real examples like this one.
The imidazole ring sits at the center—a five-membered structure made up of three carbons and two nitrogens. Numbering follows convention, where the N-H group serves as the starting point (position 1). Position 2 holds a chlorine atom, and the fourth carbon wears a nitro (NO₂) group. Placement makes a world of difference when predicting behavior and reactivity. For example, nitration at the fourth position adds a significant electron-withdrawing group, often changing how the whole molecule interacts with enzymes or receptors.
Back in the lab, I used similar aromatic compounds not just for their chemical intrigue but because small shifts in structure led to big changes in bioactivity. Medicinal chemists often tweak the imidazole ring hoping to find new antifungal or antiparasitic drugs. The presence of the chlorine atom makes this compound more electronegative and less easily metabolized, helping it stick around longer in biological systems. The nitro group’s strong electron-withdrawing character pushes the chemical into new territory of reactions. Structural changes here impact everything from solubility in water to the ability to cross biological membranes.
Any conversation about nitro compounds needs a hard look at safety. Nitro groups make molecules more reactive, sometimes pushing them toward being mutagenic or toxic. Safety authorities like the European Chemicals Agency evaluate substitutions like this one for environmental and health risks. Despite the danger, pharmaceutical researchers prize structures like 2-Chloro-4-Nitro-1H-Imidazole for their rare activity profiles, especially as starting points for new drug development.
Chemists who handle these molecules use not just gloves but a whole suite of engineering controls to ensure safety. Fume hoods, dedicated ventilation, and careful waste handling all play a part. Some labs build new derivatives by swapping groups at the 2- or 4- position, hoping to balance bioactivity with lower toxicity. These incremental changes help create more selective, less hazardous drug candidates. Regulatory bodies push the field forward by demanding more transparency and deeper research into both risk and benefit. Collaboration between academic labs, industry, and regulatory agencies has produced safer synthetic routes and waste minimization protocols that protect both workers and the environment.
In my experience, the structure of a simple-looking imidazole like this often hides a host of challenges and opportunities. Patience, careful design, and teamwork push the science forward. The beauty of chemistry shows up in these details, where just a chlorine and a nitro shift the entire fate of a molecule—for better pharmaceutical results, safer manufacturing, and more sustainable solutions down the line.
2-Chloro-4-nitro-1H-imidazole rarely gets mentioned outside chemistry circles, yet it supports fields that people rely on every day. Talking to colleagues in the lab, I’ve seen firsthand how a single compound can spark change in multiple directions. Its nitro and chloro groups open doors that a regular imidazole ring leaves shut.
The compound pops up most often in pharmaceutical labs. Chemists use it when mapping out new routes for drug discovery. One big reason: that combination of nitro and chlorine on the imidazole makes the molecule reactive in a very specific way. Medicinal chemists link it with other building blocks, shaping molecules that can fight infections or target cancer cells. It’s not a blockbuster drug by itself, but it helps researchers craft things like antifungal agents and enzyme inhibitors.
Several studies highlight that imidazole derivatives have become important in antimicrobial research. With antibiotic resistance on the rise, the world could use more inventive molecules—compounds like this often serve as scaffolds for the next batch of medicines. It speeds the journey from petri dish to pill bottle by giving researchers more options on the drawing board.
Working in organic synthesis, I noticed how often we reached for 2-chloro-4-nitro-1H-imidazole as a steppingstone. Some projects demanded molecules that respond to light; the nitro group can soak up just the right wavelengths. Others needed a starting point for ring closure reactions in pesticide development or dye manufacturing. The basic imidazole ring is easy to modify, but adding that chlorine and nitro group makes it even more interesting to chemists looking for challenging, functional starting materials.
Beyond pharmaceuticals, chemical manufacturers use derivatives like these while designing new specialty polymers and smart materials. High-performance plastics and specialty coatings benefit from compounds with strong electron-withdrawing groups. Adding a nitro or chloro group shifts the electrical properties of the final product. In the electronics industry, even small tweaks to material chemistry can improve things like conductivity or lifespan. Similar tweaks help in creating better agrochemicals—sometimes protecting crops, sometimes supporting plant growth regulation.
Anyone who uses this compound has to respect its reactive nature. Nitro groups carry risks, especially in larger quantities, since they can sometimes trigger accidental reactions. Chemical safety teams remind us that proper ventilation, sturdy gloves, and clear labeling of storage can prevent the kind of accidents nobody wants. It’s not something you find in a home workshop, and most industries keep their use tightly controlled.
Despite its widespread use in niche sectors, accessibility and safe handling matter. Training chemists in greener synthesis and proper disposal helps prevent environmental issues that came with older chemical processes. Regulatory oversight, supplier transparency, and frequent safety updates build trust from lab to factory. If creative thinkers and seasoned hands work together, compounds like 2-chloro-4-nitro-1H-imidazole will keep helping solve scientific and industrial problems while lowering risk and enhancing sustainability.
Most chemists know not to skip over storage instructions, especially for compounds like 2-chloro-4-nitro-1H-imidazole. Champions of a lab’s safety culture always stress that chemicals carrying both a nitro and chloro group demand extra respect. People working in pharmaceutical, agricultural, or specialty chemical research, including myself, have built a routine out of double-checking storage steps each time a new bottle arrives. Lax attitudes can set up a domino effect: one careless storage decision leading to degraded chemicals, or worse, hazardous situations.
This compound often ends up in synthesis labs, but its reactive nature means it calls for dry, cool, well-ventilated conditions. Humidity clouds, even from brief container openings, cause trouble. Over time, even low levels of moisture can eat away at purity. Silica gel packs stuffed in cabinets or inside bottles have saved my team’s work plenty of times, especially in humid climates.
Light protection earns its place on the checklist, since prolonged exposure can trigger unexpected changes. Even a thick amber glass bottle supports stability far better than clear plastic. For longer shelf life, temperatures around 2-8°C work well, matching the ranges you find in standard lab fridges. Leaving it out at room temperature tempts fate—decomposition and increased volatility might creep up much faster.
The compound’s reputation for causing irritation isn’t just theoretical. One lap in a fume hood with half-fast gloves leads to burning skin or a scratchy throat. Always treat gloves and lab coats as essentials, not optional. Splash goggles or face shields protect against the very real risk of a fume or dust-up.
Risk climbs during weighing or transfer. Static can toss powder around, so anti-static tools and gentle handling routines—think scooping, not pouring—help. For every step, a good fume hood keeps the atmosphere friendly, reducing the worry of lingering vapors.
In my own experience, ignoring clear label instructions on compatible materials forced hard lessons. Some colleagues once stored a batch next to strong bases, not realizing a slow reaction could start without any warning. Even tightly capped, this compound wants separation from acids, bases, oxidizers, and reducing agents. Locking chemicals in assigned zones with detailed shelf labels solves these confusion problems.
Containers have to seal tightly, no shortcuts. Old bottles with worn-out O-rings or loose caps create gaps. Leaking fumes can escape or let moisture sneak in, creating safety and quality setbacks. Resealing with new, chemical-resistant liners keeps the content in original shape for as long as needed.
Each new student or lab member deserves a walkthrough on specific storage and handling. Verbal instructions alone don’t cut it. Hands-on demonstrations and clear written procedures build good habits. Proper paperwork—safety data sheets mounted and accessible, up-to-date storage maps—builds a safety net that protects both people and research.
Routine checks help too. Schedule regular inspections for expiration dates, label clarity, and container integrity. Clean spills immediately, following clear evacuation and cleanup steps. Investing in a culture of accountability stops most mishaps before they start.
Every lab thrives on a mix of careful prep, respect for risk, and strong communication. By locking in the right storage and handling habits for 2-chloro-4-nitro-1H-imidazole, groups guard their science—and their people. Playing it safe doesn’t waste time; it preserves it, project after project.
Chemistry has always fascinated me, not because of the magic in mixing things but because of the invisible lines I figure you’re not supposed to cross. 2-Chloro-4-Nitro-1H-Imidazole fits into that group of compounds worth a second look before handling. People who work with chemicals like this can’t afford shortcuts in lab safety. Stories pile up about how a minor lapse can snowball, and just a whiff or a spill makes a bad day much worse.
The bright yellow color of these crystalline powders may catch the eye, but it hides trouble. In the lab, it’s the fumes you remember. 2-Chloro-4-Nitro-1H-Imidazole carries both toxicity and reactivity. It can severely irritate skin and eyes and the dust or vapor gets into your respiratory tract before you even realize it’s in the air. Gloves and goggles are more than recommendations—they’re your shield against chemical burns, rashes, and potential long-term consequences like asthma. Back in grad school, someone I knew skipped changing gloves and ended up with a rash that took weeks to clear. It just isn’t worth it.
The issue with many nitro-compounds, including this one, is their unpredictability. Heat and sparks can tip things over the edge. Exposed to open flames or even static, these powders may decompose and release gases you don’t want to breathe anywhere near. Handling it inside a fume hood feels much safer because proper ventilation pulls away anything airborne before it reaches lungs.
Locking bottles away in cool, dry cabinets with clear labels really pays off. I’ve seen chemicals that react with each other set off small fires in old fridges and chemical closets. Humidity and sunlight push 2-Chloro-4-Nitro-1H-Imidazole to break down faster, so isolating it from anything acidic, basic, or easily oxidized makes sense. If a bottle tips over, don’t grab a paper towel. That’s just spreading risk around. Wearing a lab coat and using a gentle, non-sparking brush to sweep up spills, then sealing the waste for hazardous disposal, might save your workspace from contamination.
Emergency showers and eyewash stations stuck in a corner get little attention until the day someone actually needs them—usually in a panic. Just last year, someone in a partner lab panicked during a splash incident. They hesitated for half a minute, then lost time trying to find the blue handle. Quick rinsing is crucial. I’ve learned to memorize low-traffic escape routes in every new building, just in case.
For inhalation or ingestion cases, there’s no room for guesswork. Reporting it fast, even if nothing feels off, keeps everyone safer. The material safety data sheet isn’t bedside reading, but on the wall near the bench it stays handy for a reason.
Every time I’ve let preparation slide, it has come back to haunt me. Double-checking that all PPE fits, labeling every sample bottle, cycling waste regularly, and setting up small runs instead of large batches can turn massive mistakes into minor hiccups. Training never gets old. Even after years in labs, refresher drills keep safety steps fresh in mind. It all boils down to everyone looking after both themselves and the next person.
Safety with tricky chemicals like 2-Chloro-4-Nitro-1H-Imidazole doesn’t just protect your own hands or lungs. It builds a culture of care. That’s worth the extra steps every time.
Chemistry doesn’t cut corners, especially in fields where small changes can make a big difference. Take 2-Chloro-4-Nitro-1H-Imidazole. Chemists use it as a key intermediate when building pharmaceuticals and specialty materials. The purity level hits home for anyone aiming for precise reactions and trustworthy data. Reliable manufacturers supply this compound at a minimum of 98% purity, and top suppliers will verify this using techniques like HPLC or NMR. High purity keeps the results repeatable, draws clear lines between experiments, and keeps clean records for regulatory purposes. In my own lab days, nothing slowed research more than having to question equipment or chemical quality. Chasing down an impurity never saved time or money.
Anything below about 98% starts to muddy the waters. Contaminants can throw off reaction yields or cause toxic byproducts—serious business when those chemicals end up in human medicines or advanced materials. For medicinal chemistry and discovery research, some teams chase every last decimal, demanding 99% or even higher to avoid rework. As an industry, pharmaceutical companies and their partners cite purity as a critical qualifying factor—not just a “nice to have.”
Chemists don’t all buy at the same scale. One group orders a gram for benchwork. Another scales up to a kilogram batch for pilot production. Suppliers have adapted by offering a range of sizes. The most common options cover 1 gram, 5 grams, 10 grams, 25 grams, 100 grams, 250 grams, and 1 kilogram. Larger custom quantities are possible but often require a discussion with the manufacturer. Each order ships in a chemically resistant bottle—usually amber glass or high-density polyethylene. Packaging matters here because this compound won’t behave well if exposed to moisture or stray light during shipping and storage.
During my experience sourcing specialty chemicals, packaging often became the only shield between a stable substance and an unusable mess. Some users might request smaller vials for quick R&D screening. Production folks need a bulk pack that won’t contaminate the next batch. Shipping rules also factor in. Regulations for this kind of compound usually require secure seals and clear labeling, no matter the volume.
Researchers are right to contact suppliers about both purity and packaging details instead of assuming all options fit their workflow. Variability in these factors can undercut an entire project, especially when the chemical steps sit at the heart of a costly synthesis. Suppliers sometimes hold batch-specific certificates of analysis, so a quick check can prevent headaches later. Seasoned chemists know that tracing quality at the source makes for fewer surprises down the line.
Anyone handling 2-Chloro-4-Nitro-1H-Imidazole faces a couple of practical realities. Safe storage means dry, cool, out of direct light—basic rules, but worth repeating after seeing a bottle ruined in a humid lab. For researchers with unique needs, some suppliers will accommodate requests for alternative packaging or tighter tolerances on purity if they’re given enough lead time. In a competitive field, that flexibility keeps projects on track. In the end, purity and packaging both have a direct impact on everything from safety to success rates in synthesis, and users benefit from suppliers willing to address those needs head-on.
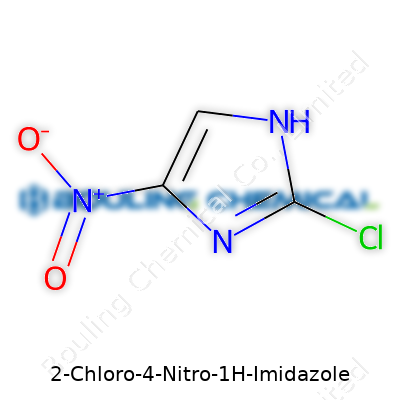
| Names | |
| Preferred IUPAC name | 2-chloro-4-nitro-1H-imidazole |
| Other names |
2-chloro-4-nitroimidazole 4-nitro-2-chloroimidazole 1H-imidazole, 2-chloro-4-nitro- |
| Pronunciation | /tuː-ˈklɔːroʊ-ˈnʌɪtroʊ-wʌn-eɪtʃ-ɪˈmɪdəˌzoʊl/ |
| Identifiers | |
| CAS Number | 3999-38-6 |
| Beilstein Reference | 136230 |
| ChEBI | CHEBI:134063 |
| ChEMBL | CHEMBL122950 |
| ChemSpider | 167358 |
| DrugBank | DB08320 |
| ECHA InfoCard | 03be5f6a-7a78-4dd4-8290-a6c1b1d1a0b4 |
| EC Number | 61927-38-8 |
| Gmelin Reference | 106490 |
| KEGG | C18509 |
| MeSH | D017859 |
| PubChem CID | 87006 |
| RTECS number | NL8925000 |
| UNII | XTQ88982C0 |
| UN number | UN3439 |
| CompTox Dashboard (EPA) | FAVOR0009753 |
| Properties | |
| Chemical formula | C3H2ClN3O2 |
| Molar mass | 163.53 g/mol |
| Appearance | Light yellow crystalline powder |
| Odor | Odorless |
| Density | 1.67 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 0.02 |
| Vapor pressure | 0.00234 mmHg at 25°C |
| Acidity (pKa) | 7.50 |
| Basicity (pKb) | 11.86 |
| Magnetic susceptibility (χ) | -55.0 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.669 |
| Dipole moment | 3.85 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 160.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -98.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -394.8 kJ·mol⁻¹ |
| Hazards | |
| Main hazards | Harmful if swallowed, causes serious eye irritation, may cause respiratory irritation |
| GHS labelling | GHS02,GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P264, P270, P273, P280, P301+P312, P305+P351+P338, P308+P311, P330, P501 |
| NFPA 704 (fire diamond) | 2-3-0 |
| Flash point | 112.4 °C |
| Autoignition temperature | 225 °C |
| Lethal dose or concentration | LD₅₀ (oral, rat): >500 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat 1330 mg/kg |
| NIOSH | NA9096000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 2-Chloro-4-Nitro-1H-Imidazole is not specifically established by OSHA. |
| REL (Recommended) | The REL (Recommended Exposure Limit) for 2-Chloro-4-Nitro-1H-Imidazole is not established. |
| IDLH (Immediate danger) | NIOSH has not established an IDLH value for 2-Chloro-4-Nitro-1H-Imidazole. |
| Related compounds | |
| Related compounds |
1-Chloro-2-nitrobenzene 4-Nitroimidazole 2-Chloroimidazole 2-Chloro-4-nitroaniline 2-Bromo-4-nitro-1H-imidazole |