Chemists have explored thiophene derivatives for over a century, and 2-Chloro-3-Methylthiophene developed a reputation on its own as early aromatic heterocycles took center stage in the search for new pharmaceuticals and materials. As synthetic organic chemistry grew more precise in the late 20th century, laboratories recognized that selective chlorination and methylation of thiophenes could create powerful building blocks. Researchers, especially after 1970, cataloged new methods of functionalization, with prominent journals detailing novel halogenated thiophenes all through the eighties and nineties. In my journey through stacks of chemical literature in graduate school, it became clear that the wider thiophene family owed its breakthroughs to curiosity about sulfur’s role in aromatic reactivity—2-Chloro-3-Methylthiophene featured in many papers as a promising intermediate.
2-Chloro-3-Methylthiophene sits at the intersection of fine organic synthesis and industrial demand. Produced and sold as a specialty chemical, this compound acts as an intermediate in agrochemical and pharmaceutical synthesis. Companies ship it in sealed amber glass for lab-scale orders, with larger quantities handled in drums outfitted for hazardous goods. The compound’s role as both a starting material and a diagnostic probe in heterocyclic chemistry gives it a solid place in research catalogs. In my own lab, requests for this compound often came from teams developing anti-infective leads or scouting new materials for organic electronics.
This molecule presents as a colorless to pale yellow liquid with a sharp, sulfur-like odor—reminding anyone uncapping it that they’re handling a thiophene derivative. Its boiling point clusters around 148 to 150°C, according to technical databooks. Solubility sits low in water, but ethanol, ether, and other organic solvents work fine. Reactivity flows from its electron-rich aromatic ring plus the activation given by the methyl substituent—with the chlorine atom creating easy entry for cross-coupling chemistry. Stability under ambient conditions is decent, though exposure to strong base or oxidizers pushes the molecule to break down or react.
Packaged with precise labeling regulations, bottles of 2-Chloro-3-Methylthiophene bear chemical identifiers including CAS number 17249-11-3, purity percentages (often above 98%), batch tracking, and hazardous substance pictograms. Safety datasheets come attached, detailing recommended storage temperatures (usually cool, dark, and dry), avoidance of ignition sources, and the need for well-sealed containers. Many suppliers validate the product via GC-MS or NMR trace, giving a spectrum or chromatogram reference to reassure labs of purity. Years in chemical supply taught me that plenty of headaches stem from poorly labeled batches, especially under rushed research timelines, so getting specifications right matters.
Production often starts with 3-Methylthiophene, which undergoes selective electrophilic chlorination. Commonly, reagents like sulfuryl chloride or N-chlorosuccinimide serve for introducing the chlorine atom. The process tends to run in polar aprotic solvents, sometimes at sub-ambient temperatures to avoid poly-chlorination or ring degradation. Recovery includes fractional distillation under reduced pressure to isolate the target isomer. Every synthesis run demands close monitoring by TLC or GC to catch side products and incomplete reactions. In the push to reduce costs, some plants scale up with continuous flow reactors, aiming for steadier conversions and safer containment of chlorine byproducts.
Chemists value 2-Chloro-3-Methylthiophene for how smoothly it accepts substitution at the 2-chloro position. Palladium-catalyzed cross-coupling reactions, like Suzuki and Stille, pave the way for new carbon bonds—key steps in building complex heterocycles, ligands, or bioactive molecules. Nucleophilic aromatic substitution with amines or alkoxides results in tailored derivatives, sharpening the molecule’s utility further. Halogen-metal exchange, using organolithium or Grignard techniques, gives another path for crafting alcohols or carboxylic acids on the ring. In my hands, small shifts in reaction temperature had outsize impact on yields and selectivity, so chemists working on scale-up keep a close eye on each degree.
The chemical world has a way of multiplying names. Researchers and suppliers list this compound as 2-Chlor-3-methyl-thiophene, or 2-Chloro-3-Methylthiofuran, and sometimes as 3-Methyl-2-chlorothiophene. In trade and patents, quick labels like CMT or CMThiophene crop up. Anyone searching for literature or regulatory data learns quickly to scan for all variants, especially when navigating international databases or customs paperwork. CAS numbers (like 17249-11-3) help cut through much of that confusion.
Standard hazard pictograms for 2-Chloro-3-Methylthiophene highlight flammability and acute toxicity, with heavy emphasis on ventilation and engineering controls for industrial settings. Spills risk environmental release, so containment and personal protective gear matter daily in the plant. Respirators, nitrile gloves, and safety goggles feature whenever open handling happens. Legal regulations in the EU and US mandate reporting by companies who import or manufacture volumes above set limits, triggering waste disposal and environmental monitoring protocols. During my times at the bench, treating even trace amounts with respect went a long way to preventing incidents and keeping audits clean.
Companies in the agrochemical sector use this compound as an intermediate on routes to new pesticides and growth regulators. Pharmaceutical groups treat it as a scaffold for antimalarial, antifungal, and antiviral drug candidates—the thiophene ring sometimes enables activity against tricky targets. Material scientists incorporate it into research on organic semiconductors, where minute tweaks to ring substitution change charge mobility and performance in flexible electronics. During tech transfer assignments, I saw the molecule’s reach extend across continents, with startup and blue-chip firms alike chasing subtle benefits from precise chemical modifications.
Active development focuses on greener synthesis routes and adaptation for automated chemistry platforms. The move to less hazardous chlorinating agents and recyclable solvents reflects both new regulations and project economics. Digital labs catalog reactivity profiles and optimize process windows with machine learning models—cutting both waste and cycle times. Researchers publish procedures that cut isolation steps or reduce purification costs, and this level of incremental improvement often drives adoption in cost-conscious pharmaceutical research. In my last job, collaborations between universities and manufacturers advanced flow chemistry hardware for better containment and reproducibility, which has started to ripple through procurement choices.
Animal studies indicate moderate acute toxicity, with LD50 values in the hundreds of milligrams per kilogram for mice by oral administration. Repeated exposure raises liver enzyme levels in test models, indicating potential cytotoxic effects on metabolic organs. Little evidence shows chronic carcinogenicity at tested doses, but lack of full long-term studies keeps regulatory bodies cautious. Inhalation of vapor or accidental skin contact can cause irritation, adding to the risk for industrial workers outside lab settings. My discussions with occupational safety experts confirm the focus on engineering controls and monitoring to protect both handlers and the environment from cumulative exposure.
Expect research to dig deeper into bioactivity screens, especially as combinatorial libraries expand the ways thiophene derivatives target infection or inflammation. Automation and data-driven synthesis may bring cleaner, cheaper routes, opening access for smaller research outfits or new geographic regions. Recyclable packaging and energy-efficient reactors could lessen the environmental burden from manufacturing. As electronic materials research matures, 2-Chloro-3-Methylthiophene’s aromatic scaffold might become key for next-generation flexible displays or sensors. New regulatory guidelines and end-of-life chemical handling procedures could change as governments set tighter thresholds for aromatic halides, pushing both industry and academia toward safer alternatives or upgraded containment.
2-Chloro-3-Methylthiophene takes the formula C5H5ClS. Each molecule carries five carbon atoms, five hydrogens, a single chlorine atom, and a sulfur atom. Stack up the weights: carbon (12.01 g/mol), hydrogen (1.01 g/mol), chlorine (35.45 g/mol), sulfur (32.07 g/mol). Add them together and you get a molecular weight of 148.62 g/mol.
My first run-in with thiophene derivatives came in a cramped university lab, the windows too sticky to open more than a crack. A professor slid over a sample with a crooked smile, mentioning its role in pharmaceuticals and electronics. I didn’t think much of it until years later, digging through notes on organic building blocks for new drug research.
In drug development, thiophene rings often make a big difference in a molecule’s flexibility and metabolic stability. Add a chlorine at the second position and a methyl at the third, and suddenly, you get a base for countless syntheses in both small-molecule drugs and specialty polymers. That distinct structure shapes how chemists design longer, more complex molecules.
Synthetic chemists value these tailored building blocks for unlocking efficiency. The methyl group tugs on the electron density, and the chlorine atom tweaks reactivity. Labs that work with antibiotics, anti-inflammatories—and recently, organic semiconductors—find C5H5ClS pretty handy.
Chemicals like this demand respect. I’ve seen safety goggles fog up in a rush, hands scrambling to reset a spill tray, all because someone skipped reviewing the MSDS. The chlorine makes this compound a little more reactive and, in some cases, a bit harsher on the skin and lungs. Working with it means handling with nitrile gloves, using a well-ventilated fume hood, and taking every label at its word. Chlorinated aromatics can carry unique risks if inhaled or absorbed, even in small quantities.
Efforts to reduce environmental load have pushed for greener solvents and tighter handling practices in labs using thiophene derivatives. Chemical recycling and responsible disposal remain patchwork in some regions. Local rules around handling and transporting chlorinated organic compounds often lack teeth unless enforced by sharp-eyed inspectors. Lab culture, not just regulation, usually keeps mistakes from snowballing into big problems.
Research teams tackle these safety and sustainability challenges head-on. Some new synthetic paths minimize by-products by choosing smarter catalysts or tweaking reaction conditions. I’ve watched a colleague switch to micro-reactor setups for fine control; it cut down on waste and almost eliminated exposure incidents.
For broader impact, manufacturers can invest in continuous flow systems and monitor emissions in real-time. Universities should double-down on safety training, not just check boxes on paper. Upstream, more companies can source precursors from suppliers who offer clear traceability and meet international environmental standards.
It comes down to open communication, sticking to strong safety culture, and staying curious about better and safer chemistry. 2-Chloro-3-Methylthiophene, like any specialty molecule, reflects both the promise and responsibility in modern science.
2-Chloro-3-Methylthiophene pops up in more labs and factories than most people realize. Stepping into the world of thiophene derivatives, this compound shows up everywhere from drug research to the creation of materials that make modern electronics possible. In chemistry circles, this molecule isn’t just another building block—it’s key for getting creative with structure and function.
Chemists looking for that extra edge in medicine synthesis often turn to 2-Chloro-3-Methylthiophene. Changing a molecule’s side chain or tweaking the core ring can turn an inactive substance into a powerful medication. This thiophene derivative is used to build larger, more complex structures during pharmaceutical development. It helps medicinal chemists add sulfur and chlorine elements into lead compounds, which can change how drugs interact with the body. Some newer anti-inflammatory compounds and experimental cancer drugs trace a part of their roots to molecules built using this agent.
When developing new drugs, researchers regularly experiment with small tweaks. 2-Chloro-3-Methylthiophene’s methyl and chloro groups provide the right chemical “hooks” for making changes that matter. In my own work, swapping out similar thiophene rings in prototype molecules sometimes gave us sharper biological activity or less toxicity—a lesson learned the hands-on way.
Crop science borrows more from pharmaceutical chemistry than you might think. A good pest control product usually needs a smart mix of stability, environmental persistence, and biological activity. Chemistry teams use 2-Chloro-3-Methylthiophene to design new classes of agrochemicals. Its structure opens the door for adding bits that help a pesticide latch onto a bug’s nervous system or make a herbicide more resistant to rain.
The need for safe, precise targeted action in the field gives 2-Chloro-3-Methylthiophene another reason to show up in research pipelines. Some commercial fungicides and insecticides were born from starting materials with this structure. Its presence isn't always obvious on the label, but the brains behind product development know its value.
Looking beyond drugs and crops, 2-Chloro-3-Methylthiophene contributes to the world of advanced materials. Organic electronics rely on the flexibility and tunable conductivity of thiophene-based polymers. Scientists adjust conductivity or color by changing the groups on the ring—methyl and chloro groups are favorites for fine-tuning. Researchers blend these modified thiophenes into new organic semiconductors. Products like flexible displays, solar cells, and advanced sensors can all trace some advances back to the use of building blocks like this.
One of my former lab colleagues worked on prototype sensors for medical diagnostics using thiophene polymers. The variations with chloro and methyl groups often provided a real difference in stability—especially when the devices had to survive outside of a clean lab.
Any conversation about specialty chemicals ends up at sustainability. 2-Chloro-3-Methylthiophene isn’t an exception. Manufacturing processes have to keep emissions and exposure in check. Chlorinated compounds sometimes raise concerns about persistence in the environment. Factories and research teams now look for safer alternatives during synthesis or better ways to break down byproducts. I’ve seen new methods that recycle or neutralize waste streams make a real difference in the environmental impact of using this compound.
Thoughtful choices in manufacturing and research can help keep both workers and the environment safer. Process improvements, closed systems, and better training remain at the front line—keeping the creative potential of 2-Chloro-3-Methylthiophene open, while cutting back on risk.
Anyone stepping into a lab or warehouse knows the sight of tightly sealed containers, warning symbols, and complex checklists. There’s a reason for all that caution. Chemicals bring real benefits to many industries, but the smallest slip in storage or handling brings risks—injury, spills, and lost product. In my time dealing with both specialty and everyday compounds, I’ve watched even seasoned veterans make mistakes when rushing or when shortcuts feel tempting. A well-organized storage space, real attention to labeling, and regular training on handling protocols mean the difference between smooth days and real disasters.
Temperature swings, humidity, and exposure to sunlight often don’t get enough attention. Most compounds behave just fine at room temperature. Some turn volatile, form crystals, or even degrade. Take hydrogen peroxide—store it warm and light hits it, concentration drops fast. Acids like acetic or sulfuric seem stable, but a corked bottle near a heat source can balloon up from gas buildup, one day devouring the shelf below. Using insulated or flameproof cabinets isn’t about ticking off a regulation box. It’s about protecting everyone who shares that workspace.
I’ve seen handwritten labels on recycled bottles, the kind that fade after a week. Good labeling includes not just the name but hazard warnings, concentration, and the date the container was filled. Some workplaces set up periodic checks. Others rely on memory—a surefire recipe for mistakes. Once, I found two nearly identical jars sitting inches apart, each with a single letter scribbled on masking tape. That episode ended hours later, with a chemist rewriting labels and cataloging every shelf. Clear, printed labels with full chemical details save time and boost safety far more than high-tech monitoring ever could.
Goggles, gloves, and proper lab coats are sometimes left hanging on a hook, especially during quick tasks. That “quick task” led to a colleague at an industrial plant suffering months of recovery after a splash from poorly stored caustic soda. Every PPE protocol gets written for a reason grounded in real accidents. Finding gloves that fit and wearing a well-maintained lab coat helps keep you from learning about chemical burns the hard way.
Pouring powders slowly, working beneath a fume hood, or storing reactive products apart from each other—these are pieces of habit built up through repetition, not just reading manuals. Using secondary containers, spill trays, and keeping incompatible compounds separated by distance or barriers proves crucial. Something as simple as segregating acids from bases prevents violent reactions, which doesn’t just protect storage; it protects people and the business itself from more than just fines or lost inventory.
Too many tragedies happen after someone forgets to check for leaks or skips a step in the protocol. Manufacturers offer guidance tailored both for stability and for safety. I’ve learned to read safety data sheets every time something new comes through the door, no matter how routine the order looks. Procedures need updating as regulations shift and as new hazards appear. Regular training refreshers, sometimes led by outside experts, help catch the little changes people miss with routine. Good storage and careful handling reflect respect for your equipment, your colleagues, and your own health.
Anyone who has worked around laboratory chemicals recognizes certain compound names immediately spark a gut response. 2-Chloro-3-methylthiophene fits squarely into this group. It’s a building block in organic synthesis, seen most commonly in the pharmaceutical sector and research labs. Its structure, marked by a chlorine atom on a methyl-substituted thiophene ring, raises eyebrows due to both the halogen and the sulfur.
Plenty of people treat substances like this as routine — perhaps too routine. That attitude often comes with experience, yet the risks hiding in subtle molecular shifts keep surfacing in accident reports. In my own lab days, careless handling of organosulfur compounds meant the whole bench reeked for days. We had a policy: don’t trust a label to tell the whole story. This one simple habit kept eyes open, no matter how “niche” the ingredient.
Looking at 2-Chloro-3-methylthiophene, volatility sits high on the list. The chemical doesn’t wait around — it evaporates at room temperature, filling the air with its distinct sulfur-laden aroma. Inhaling those vapors poses a risk. Exposure can irritate the eyes, respiratory tract, and skin. Small spills linger in the nose far longer than you’d expect. A colleague once compared the sensation to handling fresh onions mixed with diesel. That alone taught me to crack a window even for the tiniest experiment.
Spill risk isn’t just about comfort. Organosulfur chemicals, particularly those with halogen substitutions, sometimes sensitize airways and skin with repeated exposure. This isn’t theory. The Centers for Disease Control (CDC) lists thiophene derivatives among substances requiring gloves and eye protection. A single splash into the eye draws a burn intense enough to demand a trip to the emergency eyewash. Standard laboratory issue nitrile gloves stand up to short encounters, but only if changed after contact. Extended skin exposure can lead to redness or dermatitis, especially if working barehanded.
Another side of the story lands outside the lab door. Organic halogen compounds often present problems for wastewater treatment facilities. 2-Chloro-3-methylthiophene breaks down slowly in the environment, which means a careless pour-down-the-drain can stick around in waterways or soil for years. EU regulations classify many similar compounds as “aquatic hazards,” so specialized disposal companies need to handle the waste. Lab managers I’ve worked with set up satellite waste stations for just this reason, and trained everyone to label containers until pickup day.
Simple habits make the biggest difference. Lab coats, splash goggles, and gloves form a baseline. Working under a fume hood isn’t just tradition, it comes out of common sense after a single whiff of the vapor. Airflow dramatically reduces odds of inhaling the fumes — a local fire marshal once emphasized the need for annual fume hood maintenance after an incident cost thousands in cleanup.
Training can’t just be a box to check. Short safety briefings before work with unknown chemicals save real heartbreak. If a SDS (Safety Data Sheet) marks a compound for special attention, treat it that way, period. Never improvise disposal. Even tiny amounts end up traced back if pumped into shared drains. Have a chemical spill kit on hand. This mindset, coupled with consistent protective equipment, protects everyone in the workspace and beyond.
Ignoring “small” hazards always feels harmless until something goes wrong. Too many stories start with “We only needed a drop…” and end with lab shutdowns or health complaints. Long-term, the culture around handling chemicals such as 2-chloro-3-methylthiophene shapes the safety record of not only individual labs, but the entire research community. Staying vigilant means less worry, smoother experiments, and, most importantly, people going home healthy at the end of the day.
Nobody likes surprises in the lab or in manufacturing. That’s where purity grades come in. Take any widely used chemical like sodium chloride, it’s available in several grades. ACS grade, short for American Chemical Society, means you get high-purity material that meets strict benchmarks. Researchers trust this for critical experiments. Food grade, as the name suggests, passes more tests for things like heavy metals, making it suitable for food production. Then there’s technical grade, which comes at a friendlier price. These batches sometimes include more impurities—nothing that matters for industrial use, but not sharp enough for sensitive lab work.
From my own time supervising undergraduate chemistry labs, ordering the right grade made all the difference. A single slip-up led to instrument clogs, skewed test results, and a pile of wasted time. People ordering for food manufacturing or pharmaceuticals face even higher stakes. The wrong grade means more than a failed experiment; it could mean failed batches, health risks, or even recalls.
Take a walk through a supply warehouse and you’ll see everything from tiny glass vials to drums weighing several hundred kilos. Suppliers know that pharmaceutical manufacturers have different needs than a high school science teacher. For those of us running small labs or specialized setups, bottles or jars between 100g and 1kg help avoid spoilage or contamination. Larger operations prefer bags, pails, and carboys—anywhere from 5kg to 25kg and up. Some bulk buyers look for sacks or drums holding 50kg or more.
You get what you pay for: smaller packages cost more, but they prevent waste. In some fields, using a freshly opened container means more reliable results. Bulk buyers save on packaging and transport, but have to train staff on proper storage and handling.
Confidence comes from clear labeling and documentation. A reputable product comes with a certificate of analysis. This paper lists everything from the actual purity percentage (say, 99.9% for ACS grade) to heavy metal content. It also tracks the lot number and production date. If a routine test in the lab points out an inconsistency, that certificate helps trace any possible issue back to its source.
Last year, a batch of reagents meant for testing water supplies came in without documentation. The supplier scrambled to fix the mistake, but the lost time was frustrating. Labs working with medical samples cannot afford this kind of uncertainty. For industry, transparency avoids regulatory headaches and shows customers the business values safety.
Companies selling chemicals should invest in training for their teams, making sure they know the difference between each grade and why the details matter. Customers benefit from simple ordering systems that explain the choices. Digital catalogs describing purity, packaging, and applications cut down on mix-ups. Sellers that list batch numbers, purity specs, and expiration dates online win more trust.
Old habits die hard, but real investment in good training and quality systems brings accountability. Whether someone’s mixing up saline in a hospital or running thousand-liter reactors, the peace of mind that comes from accurate, well-labeled materials pays back again and again.
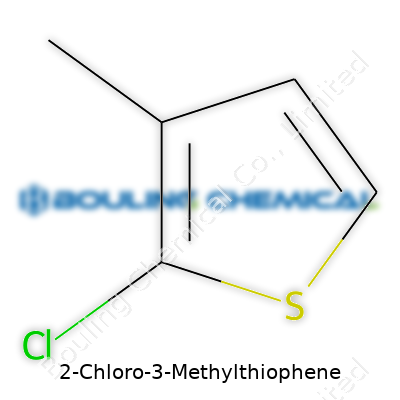
| Names | |
| Preferred IUPAC name | 2-chloro-3-methylthiophene |
| Other names |
2-Chloro-3-methylthiophen 2-Chloro-3-methyl-thiophen 2-Chloro-alpha-methylthiophene 2-Chloro-3-methyl-thiophene 2-Chloro-3-methylthiophen 2-Chlor-3-methyl-thiophen |
| Pronunciation | /tuː-ˈklɔːrəʊ-θriː-ˈmɛθɪl-ˈθaɪ.ə.fiːn/ |
| Identifiers | |
| CAS Number | 28523-23-1 |
| Beilstein Reference | 1867484 |
| ChEBI | CHEBI:38769 |
| ChEMBL | CHEMBL516786 |
| ChemSpider | 83532 |
| DrugBank | DB08295 |
| ECHA InfoCard | 100.016.694 |
| EC Number | '6923-88-6' |
| Gmelin Reference | 148888 |
| KEGG | C18801 |
| MeSH | D017220 |
| PubChem CID | 122507 |
| RTECS number | KL6825000 |
| UNII | 590LT84U3F |
| UN number | UN3437 |
| Properties | |
| Chemical formula | C5H5ClS |
| Molar mass | 146.62 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | Strong odor |
| Density | 1.199 g/mL at 25 °C |
| Solubility in water | Insoluble |
| log P | 1.98 |
| Vapor pressure | 0.9 mmHg (25 °C) |
| Acidity (pKa) | pKa = 0.7 |
| Basicity (pKb) | 13.29 |
| Magnetic susceptibility (χ) | -45.4e-6 cm³/mol |
| Refractive index (nD) | 1.550 |
| Viscosity | 1.083 cP (20°C) |
| Dipole moment | 1.75 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | S⦵298 = 329.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -4.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4922 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H226, H315, H319, H335 |
| Precautionary statements | P210, P280, P305+P351+P338, P403+P235 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | 51 °C (124 °F) - closed cup |
| Autoignition temperature | Autoignition temperature: 485°C |
| Explosive limits | Explosive limits: 1–8.6% |
| Lethal dose or concentration | LD₅₀ (oral, rat): >5000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 > 5110 mg/kg |
| NIOSH | KH8575000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.5 ppm (2 mg/m³) |
| IDLH (Immediate danger) | No IDLH established. |
| Related compounds | |
| Related compounds |
Thiophene 2-Chlorothiophene 3-Methylthiophene 2-Bromo-3-methylthiophene 2-Chloro-5-methylthiophene |