A few decades back, most people paid little attention to compounds like 2-Butyl Pyrazine—small molecules with a big impact on how things smell and taste, especially in processed foods. Pyrazines, in general, first came to notice among chemists hunting for the aromas in roasted coffee and baked bread. 2-Butyl Pyrazine’s own discovery stemmed from efforts by flavor houses aiming to replicate the nutty, roasted, and earthy notes missing from food products that had to withstand factory-scale production. The industry’s focus shifted in the fifties and sixties. Synthetic flavors offered manufacturers a way to standardize taste, improve shelf life, and keep up with the growing appetite for snack foods. Researchers published early synthesis pathways and began cataloguing the compound’s unique sensory impressions. Their work helped move this molecule from academic curiosity to ingredient status.
Producers describe 2-Butyl Pyrazine as a potent flavor ingredient that suggests toasted, nutty, and earthy tones even at low concentrations. Its typical presentation is a colorless to pale yellow liquid. In food terms, this means it can mimic or boost flavors in roasted nuts, cocoa, bread crust, and cooked rice. Aromatherapists and perfumers keep it in their toolboxes as well—pyrazines fill space with warmth where other chemicals fall flat. Most commercial samples hit high purity marks, sometimes 98% or better, since impurities disrupt its nuanced aroma. Common customers range from multinational snack brands to specialty artisan flavorists.
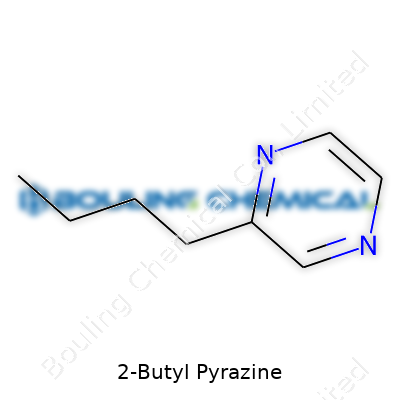
2-Butyl Pyrazine carries the molecular formula C8H12N2. Its structure places the n-butyl group at the second position on the pyrazine ring. Its boiling point lands around 191°C, with a relatively low vapor pressure. The compound dissolves in alcohol, ether, and oils better than in water. Organoleptic properties show strength: human noses catch it at low parts per billion. A direct encounter tells you why it shows up in tiny amounts in complex blends. Pyrazines, including this one, rarely break down quickly; they can persist across baking and cooking steps, giving manufacturers control over finished sensations.
Industry buyers watch for strict purity standards—sometimes 98% or higher. Reputable sellers ship it in amber glass or HDPE bottles, sealed tight and clearly labeled with batch numbers, expiry dates, and storage instructions. Panel test data, certificate of analysis, and regulatory references often sit alongside technical sheets. Safety warnings mark 2-Butyl Pyrazine as an irritant in concentrated form, recommending gloves and ventilation for anyone handling the raw molecule. Regulatory numbers, such as CAS 18138-04-0 and EINECS 242-021-2, give traceability. Flavor product codes and Sensory Descriptors flesh out its application potential.
Lab synthesis of 2-Butyl Pyrazine often starts with a condensation reaction—commonly between 2,3-butanedione and straight-chain amines under controlled temperature and pressure. Catalysts like acetic acid can speed up the reaction, though unwanted byproducts drive producers to optimize reaction times and separation methods. Extraction and distillation follow; most operators push for fractional distillation for cleaner separation. Sometimes, biotechnological pathways offer an alternative, using fermentation with engineered microbes. This method’s draw lies in “natural flavor” labeling, which some markets demand for cleaner branding. The cost, yield, and environmental impact steer R&D priorities.
2-Butyl Pyrazine keeps stable under heat and mild acids but can react with strong oxidizers and halogenating agents. Chemists may tweak the butyl group for slight shifts in aroma, stretching the product’s flavor utility. Hydrogenation isn’t a popular modification since it strips pyrazines of their distinctive aroma. Substitution on the pyrazine ring opens routes to other derivatives, each with slightly altered aromatic properties. In application, its chemical resilience means it’ll stick around through pasteurization, baking, or extrusion, holding its notes through rough processing environments where less robust molecules break down.
2-Butyl Pyrazine appears on labels under a few synonyms: 2-n-butylpyrazine, Pyrazine, 2-butyl-, or by code numbers specified by food additive regulations. Some flavor houses brand it under trade names to stake a place in proprietary blends, but the chemistry stays the same. Regulatory bodies, including FEMA and the Joint FAO/WHO Expert Committee, list it as GRAS (Generally Recognized as Safe) when used as intended.
Handling concentrates takes a little know-how. Workers in food labs run fume hoods and pull on gloves for direct handling, as the pure compound irritates skin and mucous membranes. Storage guidelines call for cool, dry spaces far from oxidizers, acids, and open flames. In finished food, the use levels drop so low the risk drops off—microgram-per-kilogram scale. Industrial producers track every batch tightly: contamination and accidental release risk recalls, especially in today’s zero-defect market. Regulatory filings require up-to-date MSDS and food grade certifications. Even the smallest supplier must log and trace every bottle—retailers and consumers alike demand it.
Snack manufacturers look for reliable brown, roasted flavors—they turn to 2-Butyl Pyrazine for its effect in crackers, breadsticks, and nut-inspired products. The chocolate industry boosts certain blends with it, trying to reproduce the complex aroma of real cocoa. Beyond food, some perfumers lean on this molecule to build warmth beneath spices or woods. Pet food flavorists use it to drive acceptance; animals seem to respond to pyrazine notes in protein-enriched kibble. Pharmaceutical excipients and even agricultural baits pull from the pyrazine chemical family, seeing value in strong, persistent scents. Every manufacturer builds a different recipe, but the backbone comes from aromatically powerful molecules like this one.
Flavor houses pour time and money into refining how they make and blend 2-Butyl Pyrazine. R&D teams tinker with fermentation methods, both for cost and for the “natural” labeling that works at retail. Sensory scientists map the aroma against consumer responses, trying to untangle why some people love “toasted” notes and others shy away. Analytical chemists refine detection limits using gas chromatography-mass spectrometry—often searching for traces in the finished product to please regulators who demand evidence of compliance. Some research chases after more sustainable process chemistry, others after more stability during storage, or less harshness in finished flavor. They keep it moving; the flavor market never stops evolving.
Toxicology work shows 2-Butyl Pyrazine safe for food use at prescribed levels. Animal studies don’t flag genotoxicity or reproductive harm at low doses, though labs set strict exposure caps. Inhalation and contact risks mean handlers need training and PPE. Long-term feeding studies in rats and mice focused on high-dose scenarios still meet approval thresholds set by regulatory agencies. The safety net rests on both acute and chronic risk analysis. If regulators ever raise concerns, it comes from overexposure or mishandling of the neat substance rather than reasonable use in products.
Many believe the path forward for 2-Butyl Pyrazine ties directly to consumer pressure for “clean label” ingredients. Bio-synthesis avenues, like engineered yeasts, attract investment as buyers lean away from petrochemical-derived flavors. Regulatory harmonization across more countries could help push it deeper into global supply chains. Sustainability asks tough questions about waste production and carbon footprint; process engineers and green chemists take their turn at rethinking how these aromas come together. In an age where plant-based and alternative protein products skyrocket, flavor designers lean on pyrazines to conjure the “cooked” and “savory” notes that make vegetables taste more exciting. If any molecule repays an investment in chemistry and creativity, it’s one like this: a little goes a long way, both in sensory value and in stimulating new approaches for safer, cleaner, and more engaging flavor experiences.
Take a stroll down any snack aisle and you’ll inhale an aroma that owes a lot to 2-butyl pyrazine. This compound brings the comforting notes of roasted nuts, baked dark bread, and earthy cocoa right to your nose. In food manufacturing, it’s one of the smallest building blocks behind the taste and smell of familiar favorites — even those “natural” flavors in chips or cereals often start life in a lab with molecules like this one.
I remember as a kid picking apart the difference between fresh popcorn and the bagged variety from the store. Bagged popcorn always smelled a little too intense, yet I kept eating it. Turns out that intensity often comes from 2-butyl pyrazine. You’ll find this compound in low concentrations in roasted seeds, nuts, and coffee beans, but the food industry adds it to products ranging from crackers to plant-based meats, all aiming for that satisfying toastiness that signals “something just came out of the oven.”
Perfume makers and flavor chemists reach for 2-butyl pyrazine because it packs a punch at extremely low doses. The smallest amount transforms bland soy protein into something just a bit closer to hamburger. It’s used in trace doses, measured down to parts per million, because the molecule quickly becomes overpowering. Use it right, and you get complexity and warmth.
The variability across foods also clues in to why producers like this molecule. A cheeseburger made with plant-based patties sometimes lacks that “grilled” feel. Add a little 2-butyl pyrazine, and it gets that earthy, nearly savory quality. It’s also one of the building blocks for “potato chip” and “roast peanut” flavors, plus a background note in some forms of chocolate. This isn’t just about taste — smell patterns trigger memories and cravings, and this compound shows up heavily in those connections.
Some folks get concerned about food additives they haven’t heard of, and that’s worth discussing. Regulatory bodies have studied the safety data on 2-butyl pyrazine for years. The Flavor and Extract Manufacturers Association lists it as safe when used as intended. It breaks down quickly in the body. Still, big food companies keep limits in place on how much they add; there’s always a temptation to crank up flavors, but the industry knows consumers pay attention to that ingredient list.
That brings up a bigger issue: why do we crave these boosted flavors? Part of it comes from the way processed food gets made. Ingredients like 2-butyl pyrazine allow snacks and frozen dinners to mimic more expensive, slow-cooked foods without the time or cost. For companies, that’s a win; for shoppers hoping to cut ultra-processed foods, those flavor tricks require extra label-reading at the grocery store.
There’s no “bad guy” in 2-butyl pyrazine by itself. The problem comes if we let snack foods loaded with lab-made flavors dominate our diets. My own experience with home cooking has me reaching more for roasted veggies and nuts to get that deep flavor naturally, but I understand sometimes budgets and schedules mean shortcuts.
At the end of the day, knowing what goes into your food — and why — brings back a bit of power. If you reach for the roasted aroma in packaged snacks, it often traces back to this single, smartly chosen molecule. Maybe that’s all the more reason to savor the toastiness you get from real cooking, too.
Everyone bumps into chemical names at some point, even if you don’t work in a lab. Food labels, flavor lists, even that new air freshener at the gas station—they all feature unwieldy names. Take 2-Butyl Pyrazine. The name sounds almost intimidating, but on paper, its formula, C8H12N2, keeps things straightforward: eight carbon atoms, a dozen hydrogens, and two nitrogens. Its molecular weight rings in at about 136.2 g/mol. Maybe that doesn’t hit you as earth-shattering, but these numbers hold the keys to everything this compound does—both the good and the not-so-good.
I remember helping out in my uncle’s bakery growing up. The punchy scents that floated through the kitchen—some of those came from compounds just like this one. 2-Butyl Pyrazine is all about aroma: a single pinch can bring out roasted, nutty, and cocoa notes. Somehow, a molecule so lightweight carries such a strong, recognizable smell. Special features in its atomic structure—those two nitrogen atoms nestled into a six-membered ring—make all the difference. The precise formula means food scientists can blend it without accidentally overpowering the whole batch.
Working in product development showed me the number isn’t just for the textbooks. The molecular weight, 136.2 g/mol, determines how the compound moves through flavorings, perfumes, and even the air you breathe. Lighter compounds can evaporate quickly, hitting your nose faster and leaving just as fast. Something like 2-Butyl Pyrazine lingers a bit longer than you’d expect, helping a roasted peanut taste “stick” on your tongue or a chocolate bar remain fragrant in its wrapper longer.
There’s a gap between the folks who measure out these chemicals and those who eat, drink, or smell the end product. After spending time in quality control, I realized how slim that gap becomes when food safety is on the line. That chemical formula isn’t just neat trivia. Regulatory bodies tie the formula to safety checks, dosage limits, and sourcing. Mess up the formula, and suddenly even a beloved coffee creamer might turn risky. Knowing the molecular weight lets companies calculate safe exposure and even helps them trace contamination sources if something goes wrong.
Reading the safety data for 2-Butyl Pyrazine, I caught myself wondering about its long-term buildup in processed foods. Most food chemists lean on benchmarks based on the formula and weight to guide their work. These numbers become decision points. Should chocolate makers stick with real cocoa or reach for a synthetic shortcut? It gets tricky when margins are thin and consumer expectations keep rising. Next time I smell something toasty and sweet—especially from a source that seems too cheap and cheerful—I’ll remember molecular weight helped make that call.
What would help most is clearer labeling, so everyday folks aren’t left hunting for a PhD just to know what’s in their food. For anyone with allergies or chemical sensitivities, formulas like C8H12N2 aren’t abstract. They spell out what’s safe and what’s risky. In my experience, the more I learned about the actual components behind flavors and scents, the more empowered I felt to question and push for safer products.
For 2-Butyl Pyrazine, the formula and molecular weight aren’t shut away in some lab notebook. They shape the way everyday choices get made, from what lands on your plate to what aromas invite you into a bakery. The details might look small, but trust me—those numbers carry real weight.
Every time you bite into a roasted nut, corn chip, or even catch a whiff of fried onions, a little bit of that toasty, earthy-aromatic magic sits behind the experience. The secret? Often, it’s a molecule called 2-Butyl Pyrazine. Scientists count it among the family of pyrazines—tiny compounds capable of carrying massive amounts of flavor. On paper, chemical names usually generate concern, but ingredients like this shape modern flavoring more than we realize.
Talking about food safety always draws my attention as a parent and someone who values quality over novelty. When I search through ingredient lists, the chemical-sounding entries stick out like a sore thumb. Food-safety regulators, including bodies like the US Food and Drug Administration (FDA) and the Flavor and Extract Manufacturers Association (FEMA), have evaluated 2-Butyl Pyrazine before it ever reached the supermarket aisle. It sits on lists such as FEMA GRAS (Generally Recognized as Safe). That didn’t happen by accident.
Backing those statuses, researchers have poked and prodded 2-Butyl Pyrazine using lab animals and cellular studies. So far, they haven’t turned up evidence that tiny exposures—like the kind used in flavoring the popcorn at movie theaters—pose a threat to health. Their evaluations consider the amounts people actually consume, not some theoretical overdose.
No safety story finishes without asking what might happen over a lifetime of eating trace compounds. A diet heavy in processed or flavored foods adds up in different ways, so the underlying risk isn't about any single molecule. Most flavor ingredients like 2-Butyl Pyrazine appear in minuscule doses measured in parts per million—sometimes even less. Compare this to other risks in daily life, and it’s a drop in the bucket. Still, some people worry about the ongoing mix-and-match of dozens of these compounds in snacks, drinks, and everything flavored to order.
Government agencies in Europe and North America have tackled this head on. Unlike synthetic colors or artificial sweeteners that entered markets with little oversight decades ago, newer flavor research follows a conservative path. Every food scientist I’ve ever interviewed swears by regulations that limit safe exposure. If new research tips the scales—if a flavor like 2-Butyl Pyrazine ever appeared risky at actual culinary doses—it would vanish from the legal list overnight.
Food runs on trust. Folks seeking total certainty about each ingredient’s safety often stick to whole foods and homemade flavors. At home, the solution means a return to basics—herbs, garlic, sautéed onions, torched nuts, and careful cooking. For those satisfied with researched safety, reading food labels and checking for reputable brands pays off. Labels won’t always show “2-Butyl Pyrazine” as a standalone line; it's folded into "natural flavors" or similar umbrella terms.
Striking balance means following new research and listening to feedback from watchdog groups, but not panicking over every unfamiliar name in ingredients lists. Vigilance means supporting ongoing research on food flavors: funding more independent studies, updating safety reviews, and fostering transparency in the food industry. Modern food science spends enormous resources keeping today’s treats safe; each of us can encourage clear labeling and regular scientific check-ins without giving up the diverse flavors that make eating enjoyable.
2-Butyl pyrazine stands out in the flavor and fragrance world, giving everything from chocolates to snacks a rich, roasted scent. For all its charm in small amounts, anyone who's had to handle bigger batches knows it’s not all sweetness. Storing any chemical, especially one with such a strong aroma and potential hazards, takes some thought and a little know-how.
Experience in small labs and larger mixing facilities taught me something fast: temperature matters. Leave 2-butyl pyrazine out at room temperature, especially on a hot day, and the odor fills the place. Even worse, high temps can break down the compound and make it a flammable headache. A dry, cool storage area well away from direct sunlight keeps things stable. In most setups, a refrigerator or a ventilated chemical storage cabinet works well. Not next to your sandwich, but not in a drafty shed either.
Some people like to cut corners with containers. Don’t. I once watched a guy pour pyrazine into an old plastic jar with a cracked lid “just for now.” By the next morning, the smell invaded every corner and the plastic lid had puckered. Go for airtight, chemical-resistant bottles or cans. Glass works if nobody’s going to knock it around. Make sure the seal fits snug, and don’t use containers that flex under pressure.
Pyrazines don’t play well with oxidizers and strong acids. I remember a close call at a job where someone stored these things together. It wasn't pretty and could have gone worse. Don’t put pyrazine near bleach, peroxides, or cleaning supplies with ammonia. Separate shelving or locked cabinets with clear labels prevent mix-ups in busy storerooms or production lines.
Handwritten sticky notes won’t cut it. Proper labeling saves headaches down the road. Use a permanent label that lists the name, batch number, storage precautions, and hazard information. OSHA and local health departments check for these things. In my experience, investing an extra five minutes on labels avoids a world of trouble—especially with powerful aromatics like butyl pyrazine.
Nobody likes gloves and goggles until they need them. Gloves—nitrile over latex—have saved my hands from chemical burns and stinky skin more than once. Safety goggles protect eyes during transfers. Store PPE nearby so nobody skips protection “just for a second.”
I’ve seen too many new hires get thrown into chemical storage without any idea why these details matter. Walk folks through safe handling, spill cleanup, and emergency numbers before letting anyone work solo. Regular refreshers keep safety fresh on everyone’s mind.
A single spill spreads fast, especially with something so aromatic. Keep spill kits—absorbent pads, gloves, and waste containers—close. Ventilate the area right away and clean thoroughly, or the smell sticks around for weeks.
Long story short, don’t treat 2-butyl pyrazine like just another flavor chemical. Respect for good storage keeps your workplace safe, stops product loss, and covers your nose from a mighty strong smell. Treat those rules like advice from an old pro who’s already learned from a few mistakes.
Biting into a roasted peanut or popping a piece of popcorn fresh out of the machine hits the senses with something instantly recognizable. That rich, nutty swirl floating up from the snack bag comes from a handful of natural compounds, but 2-Butyl Pyrazine plays the leading role. It’s the same aroma that shows up in chocolate, coffee, and even some baked goods when they’re cooked just right.
Snap-open a vial of pure 2-Butyl Pyrazine in the lab and you get smacked with a blend of toasted nuts, dark grain, and the warm, almost brown edge that gives off “just-baked” energy. It’s less sharp than some of the other pyrazines. Instead of being pungent or metallic, it delivers that mellow, earthy depth you find in peanuts or roasted bread crust. In flavor development, it’s the spark that creates “roasty” magic without adding bitterness.
There’s something simple about smell—most people remember sitting at the breakfast table, coffee drifting through the air, or taking that first handful of caramel corn at the movies. Those warm, nutty top notes, the part that smells a bit like toasted almonds or sunflower seeds, trace back to this molecule. After years of sensory testing, it’s the consistency that stands out: roasted grain, lightly earthy, and a trace of baked potato in the background. Together, it creates a sensation that feels almost homey.
Most folks outside of flavor labs never hear about pyrazines. Still, 2-Butyl Pyrazine works hard behind the scenes. In chocolate, it rounds out the bitterness of cocoa and brings up those bread-like undertones that make high-quality bars so addictive. In snack foods, even just a small bump in its concentration pushes popcorn or roasted nut aroma without needing to add tons of salt or fat.
Research out of flavor science communities backs this up. Studies published in the Journal of Agricultural and Food Chemistry highlight pyrazines—including 2-Butyl Pyrazine—as critical components in the profiles of roasted and heated foods. Recipes for dry spice blends and seasoning mixes often include “roasted” flavor notes designed to nudge the recipe in an earthier, nuttier direction by leveraging this single molecule. Without it, even carefully crafted products taste flat or strangely synthetic.
Every time new snack launches flop, frustration usually traces back to aroma. Too much 2-Butyl Pyrazine? You end up with a heavy, damp taste—almost like burnt nuts. Too little, and the food falls into the uncanny valley, missing its mark. R&D teams have learned to fine-tune levels, like adding just a pinch of spice, until they hit the sweet spot.
The big takeaway from years of experimentation: aroma molecules like this bring emotional connection. They build food memories and enjoyment. By listening to consumer feedback, reading the room during sensory panels, and trusting those first whiffs out of the oven, product developers keep chasing that real, roasted aroma that pulls everyone to the kitchen. 2-Butyl Pyrazine is just part of that, but it’s the part that makes quiet moments—like biting into warm bread or fresh popcorn—stick in memory long after the last crumb.
| Names | |
| Preferred IUPAC name | 2-butylpyrazine |
| Other names |
2-Butylpyrazine Pyrazine, 2-butyl- |
| Pronunciation | /tuː ˈbjuːtɪl paɪˈreɪziːn/ |
| Identifiers | |
| CAS Number | [18138-04-0] |
| Beilstein Reference | 1209247 |
| ChEBI | CHEBI:136533 |
| ChEMBL | CHEMBL372217 |
| ChemSpider | 145014 |
| DrugBank | DB08249 |
| ECHA InfoCard | ECHA InfoCard: 100.013.883 |
| EC Number | 25136-57-4 |
| Gmelin Reference | 113433 |
| KEGG | C12275 |
| MeSH | D014528 |
| PubChem CID | 12313 |
| RTECS number | UJ4375000 |
| UNII | 3I9D198YXE |
| UN number | NA1993 |
| Properties | |
| Chemical formula | C8H12N2 |
| Molar mass | Molar mass of 2-Butyl Pyrazine: **160.23 g/mol** |
| Appearance | Colorless to pale yellow liquid |
| Odor | nutty, roasted, cocoa, popcorn |
| Density | 0.967 g/mL at 25 °C |
| Solubility in water | Insoluble |
| log P | 1.79 |
| Vapor pressure | 0.0415 mmHg (25°C) |
| Acidity (pKa) | pKa = 2.58 |
| Basicity (pKb) | 1.4 |
| Magnetic susceptibility (χ) | -67.0e-6 cm³/mol |
| Refractive index (nD) | 1.4880 |
| Viscosity | 0.948 cP (20°C) |
| Dipole moment | 1.74 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 275.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -4322.8 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | Precautionary statements: P261, P305+P351+P338, P304+P340, P312 |
| NFPA 704 (fire diamond) | 2-2-0 |
| Flash point | Flash point: 103°C |
| Autoignition temperature | 385 °C |
| Explosive limits | Explosive limits: 1.5–13% |
| Lethal dose or concentration | LD50 (Oral, Rat): 2510 mg/kg |
| LD50 (median dose) | LD50 (median dose): 460 mg/kg (rat, oral) |
| NIOSH | SNB8050000 |
| PEL (Permissible) | PEL for 2-Butyl Pyrazine: Not established |
| REL (Recommended) | 1 ppm |
| IDLH (Immediate danger) | IDLH for 2-Butyl Pyrazine: Not established |
| Related compounds | |
| Related compounds |
2-Ethylpyrazine 2-Methylpyrazine 2,3-Dimethylpyrazine 2-Isobutylpyrazine 2-Butyl-3-methylpyrazine |