Chemists started looking for new imidazole derivatives decades ago, searching for properties that could support both pharmaceutical research and advanced organic synthesis. Among those, 2-Butyl-4-Chloro-5-Formylimidazole earned a spot due to its unique reactivity. Early laboratory notebooks recorded trials with different butyl-imidazole isomers, but adding a formyl and a chloro group brought new potential. Experienced synthetic chemists gradually figured out the steps needed to produce this compound with reliable yields and consistency, leading to broader interest in both academic and industrial labs.
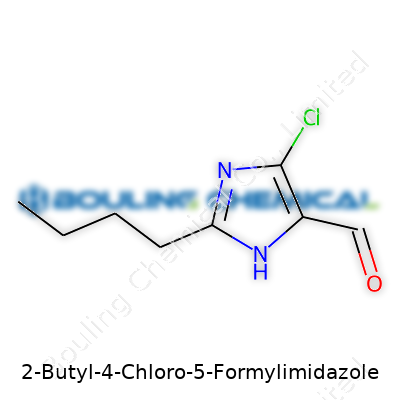
This compound features a butyl group at the 2-position, chlorine at the 4-position, and an aldehyde at the 5-position on the imidazole ring. These modifications give it properties valued for fine chemical synthesis, especially where new pharmacophores are needed. Pharmacies and research companies see this molecule as a versatile intermediate, particularly when modifying bioactive compounds or exploring new drug leads. Over the years, suppliers increased production scales and packaging options, responding to researchers eager for specialty chemicals that support curiosity-driven inquiry as well as targeted synthesis.
2-Butyl-4-Chloro-5-Formylimidazole appears as a pale yellow crystalline solid, not hygroscopic under normal laboratory storage. With a melting point around 98-102°C, it stores well at room temperature away from strong oxidizers. The compound dissolves in many organic solvents such as DMSO, DMF, and dichloromethane, yet water solubility remains low by design due to its butyl chain. The distinctive aldehyde group signals strongly in NMR spectra, making it easy for researchers to confirm compound identity or monitor reaction progress. This chemical reliably holds up during multi-step synthesis, though light and strong acid exposure can trigger slow degradation.
Suppliers typically list purity above 97% with strict batch analyses for each shipment. Labels bear CAS numbers, recommended storage temperatures, hazard pictograms, and expiration dates to help anyone in the supply chain handle it safely. Regulatory paperwork includes material safety data sheets in several languages and recommendations for waste disposal. Technicians accustomed to handling specialty intermediates will appreciate the clear lot-specific information and accessible traceability, making audits or product recalls less of a hassle.
Making 2-Butyl-4-Chloro-5-Formylimidazole depends on careful protection and deprotection strategies. In many labs, chemists start from 4-chloroimidazole, introduce the butyl group using alkyl halide chemistry, and finish with Vilsmeier-Haack formylation to attach the aldehyde selectively. Each step brings its own risks of side products, so purification by recrystallization or column chromatography often follows. Years of process improvement have lowered waste generation and increased reproducibility, trends supported by both environmental pressure and a desire for cleaner reactions. Bench chemists know how a small tweak in temperature or reagent quality can make or break a yield, and that experience drives technical advances year over year.
The reactivity of this molecule makes it attractive for coupling, condensation, and functional group transformations. The aldehyde often gets targeted for addition reactions, like the creation of Schiff bases or imines by reaction with amines. Some researchers use it in Wittig or Knoevenagel syntheses to build fully substituted imidazole cores. The chloro group opens the door to substitution by nucleophiles, supporting downstream diversification with thiols, amines, or even Suzuki-type coupling to more complex aromatic systems. Real-world examples show that even modest modifications of this parent structure can change biological activity, so many labs explore small libraries of derivatives for preliminary screening.
Scientists have sliced the name down to several shorter versions. Catalogs sometimes call it 2-butyl-4-chloro-5-formylimidazole or shorthand it as BCFi. Alternate designations from major chemical suppliers simplify ordering or inventory control by using a unique numeric identifier or skipping the “2-” prefix altogether. Watching for different names when scouring the literature matters, because older research sometimes swapped position numbers or listed it under generic halogeno-imidazole families. Supply chain transparency depends on clear, consistent labeling or researchers risk wasting valuable time or money chasing the wrong substance.
Routine lab handling requires gloves, eyewear, and access to fume hoods. Spills need immediate containment using standard absorbents. Aldehydes can irritate eyes, skin, and lungs, and the chloro group means the molecule reacts with acids or bases if mishandled. Modern suppliers ship the compound in tamper-evident containers with child-resistant closures. Chemical hygiene officers keep a close eye on labeling, ensure proper ventilation, and train staff in emergency cleanup. Safety culture now emphasizes pre-emptive risk assessment rather than waiting for a near-miss. This compound fits within that modern workflow for research chemicals.
Medicinal chemists value the structural backbone for new drug candidate libraries, especially when screening for kinase inhibitors, anti-infectives, or neurological probes. Agrochemical developers have tapped into its reactivity to build functional imidazole derivatives targeting pest control. Material scientists look for imidazole-based ligands or building blocks for advanced polymers that respond to changes in heat or pH. Laboratories working on enzyme inhibitors or molecular modeling often use this compound as a starting point due to its reliable behavior, making it a staple for both academic investigation and commercial tool compound kits.
Polished research teams probe the structure-activity relationship by swapping out the butyl or chloro group and monitoring any changes in solubility, cell membrane permeability, or receptor binding. Automated synthesis stations now streamline microgram-to-gram scale preparations, letting labs screen dozens of analogs efficiently. Computational chemists use data from real-world assays to validate predictive models based on the imidazole motif. Pharmaceutical companies work with academic partners to publish findings in high-visibility journals, keeping intellectual property secure while sharing enough data to spark fresh insights elsewhere.
Toxicological screening covers both acute and chronic exposures. Most studies show low oral and dermal toxicity at experimental levels, yet researchers continue running full panels for mutagenesis, teratogenicity, and environmental breakdown products. No significant bioaccumulation appears under normal use, but prudence calls for handling all imidazole aldehydes with respect. My own work with similar molecules has taught me how animals respond to minor changes in chemical structure, reinforcing the value of well-controlled in vivo and in vitro testing. Regulators review new data and periodically update exposure limits as scientific understanding evolves.
The next few years will likely bring stronger demand for specialty imidazoles as targeted therapies push into niches like oncology, antivirals, and diagnostics. Experienced chemists tweak production processes toward lower waste and more renewable feedstocks, matching both regulatory pressure and market demand for greener chemicals. Platforms for automated small-molecule screening support faster discovery cycles, but that only works when skilled staff keep improving the building blocks. Wider adoption may depend on integrating chemical sensors, guiding more selective reactions, or using this molecule as a reactive probe in advanced biological assays. Cross-sector partnerships help share risk and unlock new uses, making it an exciting time for anyone interested in chemical innovation and problem-solving using smart, tailored intermediates.
If you have ever wondered how the food in your pantry stays safe and free of toxins, it boils down to strict monitoring and vigilant chemical controls. 2-Butyl-4-Chloro-5-Formylimidazole fits into this story in an important way. You won’t see its name splashed across cereal boxes, but its impact touches most homes. This compound serves as a key substance in developing reagents for testing aflatoxins, which are some of the most dangerous contaminants found in grain, nuts, and processed foods.
Aflatoxins turn up wherever crops face warm, humid storage conditions—think corn silos and peanut warehouses in tropical climates. According to the World Health Organization, chronic exposure to even small amounts of aflatoxins can raise health risks, including liver cancer and stunted growth in children. With millions relying on the global food chain, labs need accurate ways to catch these toxins before food leaves the factory door.
The science behind detection comes down to a type of test called ELISA (enzyme-linked immunosorbent assay). These kits help inspectors spot dangerous levels, sometimes right on the spot, before contaminated batches reach grocery shelves. 2-Butyl-4-Chloro-5-Formylimidazole works as a building block in these test kits. Chemists use it to prepare the chemical components that bind specifically to aflatoxins, setting up a reaction that reveals their presence through visible color changes.
Back in a university lab, I helped a team run food safety checks using test kits—a hands-on introduction to these chemicals at work. We would grind up corn samples, add solutions, and watch for color shifts that signaled a problem. Working with actual protocols drove home just how fragile food safety can be without thorough testing. Even small mistakes could mean missing a contaminated batch. Seeing firsthand how much hinges on these chemical reagents brought appreciation for every link in the chain.
Manufacturers of testing kits must keep ingredients consistently pure. Impurities in raw materials like 2-Butyl-4-Chloro-5-Formylimidazole would mean less reliable results—something food producers and consumers can’t afford. Another hurdle involves access in developing regions. Labs need not just the chemicals, but trained staff and reliable infrastructure to properly screen for aflatoxins.
A step forward comes from global initiatives such as the FAO’s food safety projects that supply training, protocols, and sometimes subsidized kits to communities facing frequent contamination. Agencies and universities work together to make certified reagents widely available, and though progress can move slowly, every improvement helps prevent illness.
Investing in local production of key chemicals like 2-Butyl-4-Chloro-5-Formylimidazole could bring prices down and improve supply chains. Science education also plays a major role—when more technicians can run effective tests, fewer dangerous shipments slip through. Collaboration across countries and sharing innovations openly ensures food safety remains more than just a buzzword.
Reliable food testing depends on trusted building blocks. 2-Butyl-4-Chloro-5-Formylimidazole might seem obscure, but its role in protecting global health keeps it on the radar for chemists and regulators alike.2-Butyl-4-Chloro-5-Formylimidazole stands out for its combination of a butyl group, a chlorine atom, and a formyl group attached to an imidazole ring. Its molecular formula, C8H11ClN2O, captures this arrangement. The count comes from eight carbon atoms, eleven hydrogens, one chlorine, two nitrogens, and a single oxygen atom. A researcher can determine its molecular weight by summing the atomic weights: carbon (12.01 g/mol), hydrogen (1.008 g/mol), chlorine (35.45 g/mol), nitrogen (14.01 g/mol), and oxygen (16.00 g/mol). Running the math, the molecular weight lands at about 186.64 g/mol.
Every element in the formula influences how the molecule behaves. The butyl group gives the compound some flexibility and nonpolar character. Chlorine, being electronegative, changes electronic distribution and can tweak the reactivity—especially in ways that matter for organic synthesis. The formyl group turns the molecule partly aldehydic, which brings new types of chemical reactions into play.
I've seen firsthand how knowledge of molecular weight shapes the planning of lab experiments, not just in major research but in college chemistry too. Say, a scientist needs to prepare a specific concentration for a reaction. They turn to the molecular formula and weight for accurate mixing. If the calculation is off, purity, yield, and ultimately the reliability of the experiment are at stake. Accurate data drives outcomes in these situations.
Pharmaceutical chemists care about such compounds because the imidazole ring often forms the core of drugs that target enzymes and proteins in the body. The chlorine atom can affect how well a drug candidate binds to its target or moves through the body. A simple difference—one atom swapped for another—can make or break the success of a whole research project. C8H11ClN2O isn't just a formula; it’s a blueprint for how this compound fits into the bigger picture of medicinal chemistry and drug design. That’s why accurate knowledge, based on reliable calculations, underpins the trust in published data and peer-reviewed research.
In graduate research, mistakes in molecular weight can mean wasted months and resources. Poor records or mislabelled bottles let down everyone involved. Reproducibility—a cornerstone of experimental science—starts with getting the basics right. In my work, a single data error meant backtracking through weeks’ worth of logs; the headache taught me to always double-check foundational numbers. Small details demand attention because they ripple out and affect every step after.
These days, open access chemical databases and digital lab notebooks reduce the odds of careless copying or outdated references. Modern chemical informatics tools let me enter a structure—either by drawing it or using a SMARTS/SMILES string—and instantly check synonyms, weights, and hazards. Resources like PubChem and ChemSpider provide trusted, peer-reviewed data so researchers don’t end up using conflicting sources.
For students, clear data and simple presentation help build a working knowledge of how structures relate to properties. For advanced teams, consistency keeps reporting and auditing transparent. That’s not abstract theory; it’s about honest communication and shared progress.
Chemical storage looks like a technical topic, but it comes down to sensible steps and a dose of caution. If you’ve ever worked in a lab, you know certain reagents can bite back if left unchecked. 2-Butyl-4-Chloro-5-Formylimidazole isn’t the most dramatic on the shelf, but like most imidazoles, it deserves respect. Proper storage isn’t just a box to tick; it protects people and quality alike.
Some chemicals can take a bit of heat and humidity, others fall apart fast. 2-Butyl-4-Chloro-5-Formylimidazole needs a cool, dry environment. Direct sunlight or warmth speeds up chemical changes and, as I’ve seen before, even a few degrees above recommended temps can boost impurity levels—or, worse, set off reactions no one wants. I always recommend aiming for storage between 2°C and 8°C, typical fridge conditions. Going colder, like in a freezer, may bring on crystallization or cause the bottle to break, which solves no one’s problems. Warm rooms, hot storerooms, or sunlit benches risk ruining an expensive compound.
Humidity doesn’t help either. Moisture in the air can sneak through loose lids or weak packaging and hydrolyze the formyl group, changing what’s in your bottle. I’ve seen bottles turn sticky or cake up inside—clear signs humidity’s left its fingerprint. Dry conditions matter. Use desiccators or even silica packs if your setup lets you. The best packaging keeps out both air and water; glass bottles with airtight seals outlast plastic and stand up to temperature shifts better.
Chemicals don’t like company if the neighbor’s a bad match. Pairing 2-Butyl-4-Chloro-5-Formylimidazole with strong acids, oxidizers, or bases brings unnecessary risk. Stories circulate of shelf accidents—one misplaced acid bottle, and suddenly sensitive chemicals boil, discolor, or burst their seals. Pay attention to labeling and store separately: an organized shelf is a safe shelf.
Good habits make a difference. Lab life taught me never to trust forgotten bottles, faded labels, or leaky caps. Permanent, chemical-resistant labels save time and keep everyone honest about what sits inside a container. Expiry dates may sound routine, but ignoring them turns cleanup day into a guessing game. Regular checks on seals, bottle clarity, and residue cut down on both error and waste.
Old or degraded 2-Butyl-4-Chloro-5-Formylimidazole needs a plan. Don’t pour it down a drain or toss with regular trash; waste rules for halogenated compounds run strict and for good reason. Separate containers for disposal and clear protocols stop minor slips from becoming environmental or legal headaches. Share lessons—colleagues appreciate short reminders far more than lengthy incident reports.
Careful storage isn’t overkill; it’s a simple safeguard that I’ve seen prevent costly mistakes. By minding temperature, humidity, chemical neighbors, and labels, labs and storerooms can dodge disasters before they happen. In the end, safe handling lets scientists focus on discovery—rather than damage control.
Ask anyone with hands-on lab experience, and they’ll tell you, some chemicals you just treat with more respect. 2-Butyl-4-Chloro-5-Formylimidazole carries a complex name, but it’s earned a spot under a magnifying glass for good reason. To really get what this compound means for safety, health, and the environment, you need to look at how it behaves, who encounters it, and what happens if it escapes into the wider world.
From what chemists and toxicologists have found so far, there’s not much room for complacency. Hazards linked to 2-Butyl-4-Chloro-5-Formylimidazole stem from the imidazole ring itself—this isn’t some benign additive or food-grade substance. Many compounds with formyl (aldehyde) groups or halogen atoms can be irritating to skin, eyes, and respiratory systems. Breathing in dust, vapor, or simply splashing a concentrated solution can trigger chemical burns and irritation. Animal studies point toward acute toxicity when taken in high doses, with effects ranging from liver and kidney stress to neurological disruption.
Having handled unknowns on the bench, I can say safety gear isn't there for show. Gloves, goggles, and a solid fume hood give the first layer of protection. Chemical companies require training for all staff handling materials like this, and the rules exist for good reason. Exposure limits don’t float out of thin air. Safety data sheets for similar compounds often come stamped with GHS hazard statements, like “causes serious eye irritation” or “harmful if inhaled.” Chronic exposure can lead to bigger issues—cumulative toxicity is a real worry, especially for people working with solvents or reactive intermediates as part of daily life.
Environmental persistence can turn a lab hazard into a community risk. Some halogenated chemicals stick around in soil or water much longer than anyone would like. What happens downstream matters as much as what happens inside a building. If not managed well, run-off or airborne particles can hurt wildlife or contaminate local water supplies. Given what’s known about similar compounds, proper storage, disposal, and spill response prevent ugly problems later.
Lab managers and chemical buyers carry real responsibility here. Substitution sits high on the list: if a less-toxic alternative does the trick, it makes sense to switch. If using 2-Butyl-4-Chloro-5-Formylimidazole remains absolutely necessary, strong containment, airflow management, and clear labeling bring down exposure. Training keeps people alert—fast thinking stops accidents from becoming disasters. Companies ought to follow up with regular health monitoring for staff and routine checks for leaks or improper disposal. For folks outside research or industry, stricter labeling and public access to data give everyone a chance to speak up for safer handling.
Trust in any chemical only goes so far as the safeguards in place. The evidence draws a line—2-Butyl-4-Chloro-5-Formylimidazole shouldn’t circulate freely in public spaces, and even skilled users treat it with care. Staying sharp, following protocols, and pushing for safer alternatives matter just as much as clever science in the real world.
People who work with chemicals like 2-Butyl-4-Chloro-5-Formylimidazole know just how important it is to stay a step ahead of the risks. This compound, often found in chemical research labs or manufacturing sites, carries real hazards. I spent years in a university chemistry lab, and every new substance brought its own safety conversations. Some had immediate dangers, some were more sneaky, building up harm over time.
Folks handling this compound should remember that direct skin contact, inhalation, or accidental ingestion can all cause harm. Some imidazole derivatives have shown toxic effects in lab animals, and personal protective gear isn’t just a formality—gloves, goggles, and a quality lab coat mean the difference between a typical day and an emergency room visit. I've witnessed enough chemical splashes to know even experienced workers can get complacent and pay for it later.
Storage and handling define much of lab safety. 2-Butyl-4-Chloro-5-Formylimidazole should always sit in a tightly sealed, clearly labeled container. Flammable or moisture-sensitive impurities can sneak into a poorly sealed bottle, causing unexpected reactions. I always double-check for leaks and make sure containers stay stored away from heat and direct light.
Before opening anything, a splash shield belongs between you and the reagent. Chemical fume hoods have proven their worth in my own work; they pull toxic fumes away before anyone can breathe them in. Keeping chemicals on sturdy, tip-resistant shelves and having spill kits within reach can make all the difference if something goes wrong.
Throwing hazardous chemicals down the drain hardly ever ends well. Wastewater systems can't break down complex substances like 2-Butyl-4-Chloro-5-Formylimidazole. They end up polluting rivers, harming wildlife, and sometimes even making it back into drinking water. Municipal fines do not compare to the guilt that comes from knowing you helped poison a waterway.
Disposal always starts with talking to your waste contractor or local hazardous waste program. Containerize unused or leftover material in a compatible, leak-proof vessel, and label the contents with the full chemical name and hazards. Chemical neutralization or incineration in a controlled facility often works best for imidazole derivatives, but only trained professionals should attempt either process on this scale.
Safety training has a reputation for being boring, but real stories of near-misses and accident reports stick with people much better than rule recitations. I advocate for hands-on drills. Practice spill cleanups and emergency responses before disaster strikes. Encourage every worker, from new interns to seasoned PhDs, to speak up if they spot sloppy habits.
Safety data sheets hang on the wall for a reason. They change with new research, and regular review helps everyone stay up to date. Involving environmental health and safety officers in routine lab walkthroughs often exposes problems before they become incidents.
Responsible chemical use protects not only the people in the room, but also the broader community. Smart handling of 2-Butyl-4-Chloro-5-Formylimidazole means anticipating dangers, investing in proper equipment, and choosing safe, professional disposal every single time. Choosing caution doesn't just save face—it saves lives and keeps research moving forward without compromise.
| Names | |
| Preferred IUPAC name | 2-butyl-4-chloro-5-formyl-1H-imidazole |
| Other names |
BCF 2-Butyl-4-chloro-5-formyl-1H-imidazole |
| Pronunciation | /tuː-ˈbjuː.tɪl-ˈklɔːr.oʊ-ˈfaʊr.mɪl-ɪˈmɪd.əˌzoʊl/ |
| Identifiers | |
| CAS Number | [7341-24-4] |
| 3D model (JSmol) | `3DModel_jmol("C[C@@CC1=NC=C(N1)C=O]Cl")` |
| Beilstein Reference | 136703 |
| ChEBI | CHEBI:63629 |
| ChEMBL | CHEMBL143541 |
| ChemSpider | 10673780 |
| DrugBank | DB08325 |
| ECHA InfoCard | 18e6e2f3-6618-4b8f-8e53-68bd9416c620 |
| EC Number | 613-130-8 |
| Gmelin Reference | 79268 |
| KEGG | C18208 |
| MeSH | D017317 |
| PubChem CID | 72537 |
| RTECS number | UW2275000 |
| UNII | 9P783446MP |
| UN number | UN3438 |
| Properties | |
| Chemical formula | C8H11ClN2O |
| Molar mass | 155.61 g/mol |
| Appearance | White to light yellow crystalline powder |
| Odor | Odorless |
| Density | 1.26 g/cm³ |
| Solubility in water | Insoluble in water |
| log P | 1.99 |
| Vapor pressure | 0.000016 hPa (25 °C) |
| Acidity (pKa) | 7.20 |
| Basicity (pKb) | 12.19 |
| Magnetic susceptibility (χ) | -62.3×10^-6(cm³/mol) |
| Refractive index (nD) | 1.553 |
| Viscosity | 1.06 mPa·s (25°C) |
| Dipole moment | 3.73 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 385.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -70.5 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -5706 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS05 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | Precautionary statements: P261, P264, P271, P272, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P321, P330, P332+P313, P333+P313, P337+P313, P362+P364, P403+P233, P501 |
| NFPA 704 (fire diamond) | 1-3-0-ない |
| Flash point | 134.6°C |
| Autoignition temperature | Autoignition temperature: 485°C |
| LD50 (median dose) | LD50 (median dose): >5000 mg/kg (rat, oral) |
| NIOSH | DJ1050000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 2-Butyl-4-Chloro-5-Formylimidazole is not specifically established by OSHA or other major regulatory agencies. |
| Related compounds | |
| Related compounds |
Losartan Imidazole 2-Butyl-4-chloro-1H-imidazole-5-carbaldehyde 2-Butyl-4-chloro-5-formylimidazole hydrochloride |