Chemists in the mid-20th century focused plenty of attention on pyrazines after a string of discoveries around flavor compounds in cooked foods. A fascination with why roast coffee and bread have their signature aroma drew more than a few scientists to molecules that could mimic those subtle, evocative notes. It didn’t take long before 2-Butyl-3-Methyl Pyrazine popped up as a standout for its distinctly nutty, earthy character. Laboratories intently sought ways to not just isolate but also synthesize these aroma compounds. For years, production often relied on labor-intensive extraction from roasted and toasted foods. The game changed as industrial synthesis caught up, transforming 2-Butyl-3-Methyl Pyrazine from a culinary curiosity into a commodity. Steady improvements have only sharpened the focus on purity and economic yield, prodded along by the growing popularity of convenience foods and artificial flavor enhancement.
Walking into a flavor house, it’s rare not to bump into 2-Butyl-3-Methyl Pyrazine, at least metaphorically, hiding in some glass vial. This compound doesn’t shout; it quietly rounds out product lists for those crafting savory blends. Used mainly as a flavoring agent, it finds a home in snacks, cereals, chocolates, and even tobacco products, thanks to its deeply nutty, raw potato, earthy aroma. It’s available commercially as a colorless to pale yellow liquid, supplied in tightly sealed drums or smaller amber bottles, each stamped with precise labeling. Nearly every supplier leans into the purity angle, boasting values that typically top 98%, which matters a lot for companies aiming to stick within regulatory bounds.
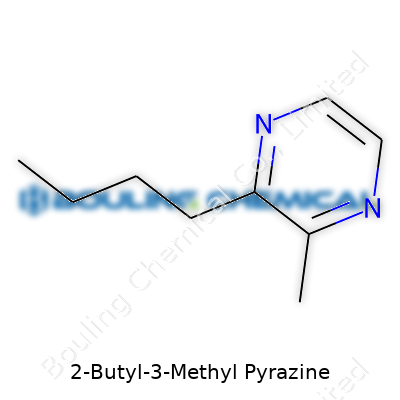
This compound holds its own in a lineup of aromatic chemicals. Structurally, it’s a pyrazine ring bearing a butyl group at the 2-position and a methyl at the 3-position, which adds just enough steric heft to shape its boiling point—hovering around 188 to 190°C at atmospheric pressure. It doesn’t mix easily with water but blends right into fats and oils, exactly where most flavor systems want it. Its density hovers just under 1 g/cm³, and its refractive index typically comes in just above 1.48. With a flash point that sits above 60°C, storage isn’t as tricky as with some lighter spirits, though its low volatility does mean it tends to hang around once in solution.
Regulators take a close look at how flavor and fragrance chemicals are labeled. Suppliers list chemical abstract numbers (CAS 35786-04-8), purity percentages, and sometimes even GC chromatograms to back up those numbers. Labels mention its use for industrial or research purposes, never for direct human consumption outside tightly defined limits. Often, one finds storage guidance—cool, dark, and dry settings—and safety icons for combustible liquids. Full disclosure of possible allergens or synthesis byproducts helps buyers navigate modern labeling requirements, while batch numbers and manufacturing dates help with traceability. Such attention to detail keeps manufacturers honest and ensures end-users can troubleshoot if anything drifts off specification.
Producers usually stick with chemical synthesis over natural extraction, given the challenges in pulling enough from food sources. One popular method involves condensing substituted butylamines with α-diketones under controlled conditions—modern tweaks to classic pyrazine syntheses. Timing, temperature, and choice of solvent all shape the resulting purity. Recrystallization and vacuum distillation follow, both aimed at scooping out any contaminants. Regulatory shifts recently forced manufacturers to clean up their act, leading to tighter controls on reactants and waste handling. The technical route isn’t particularly glamorous, but process engineers know that shaves a few cents off per kilo when scaled right.
2-Butyl-3-Methyl Pyrazine puts up a decent fight against mild oxidizers, though stronger reagents can break open its aromatic ring. Chemists seeking analogs or derivatives often try alkylation or acylation, hoping to unlock new aroma notes or solubility tweaks. Hydrogenation won’t reduce the pyrazine ring under standard conditions, but higher pressures and specialized catalysts can open up new doors—sometimes literally, on the lab bench. Still, every modification means starting over with toxicity and sensory testing, a costly and time-consuming path that doesn’t always pay off for ingredient firms. Yet, fine-tuning its structure produces a long list of cousins, each with its own application sweet spot, especially in flavor modulation.
Browse technical catalogs, and this compound shows up under several guises. It’s often listed simply as BM Pyrazine, or sometimes as 2-Butyl-3-Methylpyrazine, with or without hyphens. Less formally, flavorists might shorthand it to Butyl Methyl Pyrazine. International buyers sometimes use translations or local codes, adding minor confusion when sourcing globally. For regulatory documents, the IUPAC name prevails, though trademarked blends and proprietary mixtures may only hint at its presence. A sharp eye and CAS number help avoid mix-ups, especially in busy procurement offices.
This compound doesn’t draw much drama in small doses, but it doesn’t give carte blanche for cavalier handling either. Standard practice calls for gloves, goggles, and adequate ventilation any time open volumes are used. Spills may irritate the skin, and vapor concentrations, while difficult to reach under normal conditions, can cause headaches or dizziness if inhaled. Occupational exposure limits err on the conservative side, thanks to the lack of conclusive chronic toxicity data. Storage guidelines recommend keeping it away from flames or high heat, and disposal rules urge treatment as a hazardous organic. Staff training sessions stress quick cleanup and careful bottle handling, shaped by years of lessons learned in busy production areas.
Snack technologists reach for 2-Butyl-3-Methyl Pyrazine to add roasted or nutty top notes to everything from potato chips to breakfast bars. Makers of meat substitutes add a dose when chasing authenticity without actually cooking real meat. Chocolatiers use it to round out the flavor profile of dark chocolates, especially at lower-quality price points where the bean’s own aroma isn’t enough. Tobacco blenders include it to deepen complexity while masking less pleasant off-notes from fillers. Toothpaste flavor houses, more rarely, experiment with it for earthy notes in specialty blends. It even finds a role in certain perfumes, deployed in trace amounts to anchor compositions with woody, root-like facets.
Research pushes boundaries on two fronts: analytical and application-driven. Analytical chemists keep improving detection and quantitation methods, so end products remain within legal sensory thresholds. Food scientists experiment with its stability during baking and extrusion, since some flavor molecules break down, but 2-Butyl-3-Methyl Pyrazine sticks around longer than many, making it reliable in processed foods. R&D efforts increasingly look to bio-based synthesis, though current methods remain largely petrochemical-derived. Academia teams up with industry, sharing compound libraries and sensory data to see which structural tweaks produce new effects. Regulatory pressure, combined with consumer preference for “natural” flavors, nudges even more research into alternative routes carved by yeast or bacteria—though these methods rarely make economic sense yet.
Toxicologists want hard numbers: acute oral and dermal LD50 values, chronic exposure impacts, metabolism details, allergenicity questions. Animal studies show that this compound carries relatively low acute toxicity, with minor irritation at full strength but few systemic risks at flavoring doses. Chronic testing, especially focused on reproductive and developmental toxicity, still leaves small gaps—a consequence of limited long-term data in humans. European and American agencies keep lists up to date, revising allowable daily intake guidance as more studies surface. Experienced flavor chemists stay within conservative guidelines, even if toxicologists increasingly view the risk profile as low in most food contexts.
There’s a growing appetite for authentic, full-bodied flavors as consumer tastes evolve beyond bland, mass-market snacks. This trend shines a spotlight on pyrazines, especially those able to punch above their weight in small doses, like 2-Butyl-3-Methyl Pyrazine. Regulatory trends bend toward “natural” labeling, so future work likely steers toward fermentation-based production or novel plant cell factories. Technical hurdles, like precursor supply and enzyme specificity, still complicate these dreams, but progress seems steady, not just wishful. Ongoing advances in analytical chemistry and molecular gastronomy inspire product developers to fine-tune blends and pursue more nuanced sensory impacts. The odds are good that the food—and even fragrance—world will see more of this quietly impactful molecule, given the momentum of market demand and technical innovation alike.
I still remember the look of confusion at a dinner table when I mentioned 2-Butyl-3-Methyl Pyrazine. Most folks had no idea it even existed. Surprise, it’s not some exotic cleaning agent or industrial chemical. This compound, found naturally or made in labs, pops up in a place most of us enjoy: food. You bite into some potato chips or dig into a roasted nut mix, and you taste hints of toastiness or roasted notes. That’s pyrazine at work, doing an important job in food science.
If you have ever wondered why some snacks pack that irresistible roasted or “moreish” punch, it often traces back to 2-Butyl-3-Methyl Pyrazine. Food scientists use this molecule to boost nutty, earthy, and roasted flavors in everything from corn chips to cocoa. Flavor is huge in the world of packaged snacks, where repeat sales depend on creating a taste you want again and again. This compound lands in cheese crackers, roasted nuts, and certain breakfast cereals because it tricks the mind into thinking food was roasted fresh. Even in trace amounts, it changes the game, turning bland flour into something that reminds folks of a campfire or skillet.
The compound occurs naturally in roasted vegetables, coffee, meats, and nuts, but there’s never enough from nature alone to flavor an entire batch of snacks. Sourcing the compound synthetically means manufacturers can keep the popular roasted notes consistent, no matter the season or harvest. The food industry leans on this kind of chemistry not just for flavor, but to replace or amplify what’s already present. It’s a little like magic, though it does raise questions about transparency and consumer awareness.
Not many people talk about it, but pyrazines, including 2-Butyl-3-Methyl Pyrazine, also shape the world of scents. The perfume industry often borrows molecules that wake up the senses or set a mood. This one goes into earthy, “green,” and even slightly nutty perfume profiles. Walking through a fragrance store, if a scent reminds you of sun-toasted bean fields, you’ve likely encountered this molecule.
Aromatherapists and some niche product makers tap into its “real” aroma, using it to evoke specific moods or memories. It’s subtle and often blends into complex fragrance recipes, but to a seasoned nose, those green and roasted hints stand out.
Plenty of folks feel uneasy hearing about “synthetic flavor compounds.” The phrase alone makes some shoppers turn around in the grocery aisle. Still, it’s worth noting the compound appears in small amounts and passes regulatory reviews before hitting supermarket shelves. Studies haven’t linked these low doses to health problems. Still, a real debate continues over how to label additives and whether people are getting enough information to make their own food choices. Trust matters here.
There’s an opportunity for clearer ingredient lists and better communication. Back in my own kitchen, cooking with a good skillet and fresh nuts still gives the best roast flavor. But in a world where food is often made a thousand miles away, chemistry like this bridges the gap. The flavor stays fresh, shelf life goes up, and snacks retain personality week after week. Balancing those benefits against full transparency and preference for natural ingredients keeps the conversation about food chemistry both lively and important.
Anyone who’s spent time poking around in a kitchen or worked in a food lab learns that not every flavor comes straight from the spice rack. Some of the most powerful tastes come from odd-sounding chemicals, and 2-Butyl-3-Methyl Pyrazine proves this point. Every time a potato chip delivers a roasted, nutty punch, chances are a pyrazine is involved. This particular molecule can trick your brain into thinking there’s toasted grain, baked bread, or earthy coffee hiding in a food. Take a whiff, and you’ll catch something you can only describe as unmistakably “roasted.” Not burnt, not raw, but a golden-brown, toasty quality that signals comfort food.
Bakers and snack makers rely on these nuances. People can taste roasted grain notes in breakfast cereals even before milk hits the bowl. Open a pack of roasted nuts, and the deeper, almost chocolate-like, savoriness comes into play. The way 2-Butyl-3-Methyl Pyrazine blends those dark-edged, slightly green, almost hay-like flavors brings life to foods that need a punch of realism. Nature uses the same molecules—roasted coffee, baked bread crust, sun-warmed seeds—many of those warm, homely food smells tie right back to this family of molecules.
If you’ve sampled a variety of foods and found yourself loving the complex aroma of grilled vegetables, you’ve already brushed up against this flavor compound. The answer doesn’t come from the grill alone. Food scientists turn to 2-Butyl-3-Methyl Pyrazine to punch up that roasted reality in snacks, instant soups, crackers, or even some savory marinades. These tweaks are subtle, but they make a difference. Toss a handful of plain cornflakes in the oven, and they get more aromatic and comforting—not from caramelized sugar, but from new molecules popping up as the grains brown. This change is often the result of pyrazines.
To get down to the flavor itself, it brings out roasted hazelnut, fresh-baked bread, and maybe a hint of roasted bell pepper or popcorn. Not every nose catches it the same way, but the dry, grainy, lush toastiness feels familiar. Food developers love it because people love it. Most snacks you remember fondly, from nut mixes to certain chocolate bars, owe a good chunk of their irresistible taste to the way pyrazines round off sharp edges and add depth.
As more people hunt for plant-based snacks, flavorings like 2-Butyl-3-Methyl Pyrazine let brands mimic the roasted satisfaction found in old-fashioned comfort foods. Some shoppers worry that “flavor compounds” mean artificial shortcuts, but it’s not so black and white. Many of these molecules occur naturally during roasting or baking—food makers simply borrow from nature and tune flavors up or down. Snack companies count on these molecules to create craveable flavors without tons of salt or fat.
The next time you bite into something with a rich, cereal-like crunch or catch a waft of roasted vegetables in a packet of soup, take a second to notice what’s going on. There’s real know-how behind that sensation—simple molecules like 2-Butyl-3-Methyl Pyrazine making everyday treats taste more like home.
Anyone who reads the small print on packaged food probably comes across more tongue-twisting terms than they care to count. 2-Butyl-3-methyl pyrazine is one of them. This compound pops up in ingredient lists because it gives off an earthy, roasted, nutty aroma. It doesn’t come from a mystical rainforest or a lost island; chemistry labs make it, then food firms add it to snacks, sauces, and other processed products. What does it do? It helps potato chips taste toastier and coffee drinks taste more like coffee.
Before adding anything to a food label, producers have to clear regulatory hurdles. The U.S. Food and Drug Administration recognizes 2-butyl-3-methyl pyrazine as "generally recognized as safe" (GRAS). This doesn't come from a roll of the dice. Scientists pore over studies and data before labeling a food additive this way. Organizations in Europe and other countries also include similar versions of this compound in their lists of permitted substances.
It’s easy to get spooked reading chemical names. A few years back, I decided to dig into research about flavor additives after seeing a scary-sounding name on my favorite granola bar. I found that most research on 2-butyl-3-methyl pyrazine looks for links to toxicity, allergy, or cancer risk. Published studies give the same answer: at the small doses used for flavoring, the compound doesn’t cause harm. The flavor industry typically uses it in microgram or milligram levels. For anyone worried about buildup, studies show it breaks down quickly and leaves the body.
Plenty of folks worry about “artificial” anything in food. The fear isn’t always baseless. Food history holds a few cautionary tales—think about additives banned years after hitting the market. The lesson seems simple: don’t ignore caution, even if regulators approve something for now. What concerns me is the lack of transparency about how much we eat. Walk down a supermarket aisle, and you’ll see flavor compounds in almost everything processed. Add them all up, and nobody can really say how it all interacts in the human body over a lifetime.
It’s easy to point fingers at “chemicals in food” and demand bans, but the story isn’t so black-and-white. We lean on science for answers, so better transparency would help people make choices based on facts, not feelings. Companies could publish regular updates about the safety research on these ingredients. Regulators could require clearer labeling about amounts used, not just names. For anyone who wants to avoid additives altogether, whole food diets steer around these questions.
Instead of demonizing every unfamiliar name, we should keep a close eye on new research and update rules quickly when new risks show up. Meanwhile, a little healthy skepticism mixed with fact-based reading goes further than wild headlines. Safety doesn’t mean risk-free, but with the data we have, small quantities in food seem reasonable for now. Anyone still uneasy always has the choice to check labels or go for food with short, familiar ingredient lists.
Picture biting into a roasted peanut or a warm slice of bread. That deep, nutty, toasted kick comes from molecules that usually don’t get much love outside chemistry labs. 2-Butyl-3-Methyl Pyrazine is one of those behind-the-scenes workers, lending bold notes to all kinds of food, from savory snacks to sweet treats. This isn’t just filler — it gives snacks that roasted character people crave.
The punch comes in a tiny package. In most snacks or flavorings, recommended usage levels for 2-Butyl-3-Methyl Pyrazine range between 1 to 10 parts per million (ppm). To put that in perspective, a million drops of water would only need a handful of drops of this pyrazine to make an impact. Start going above 10 ppm and you risk an over-roasted edge nobody asked for.
Chefs and food technologists rarely reach for higher doses. They know there’s a sweet spot — go light or ruin the batch. The FDA and EU regulations treat 2-Butyl-3-Methyl Pyrazine as “generally recognized as safe” (GRAS) when used within accepted limits. This guidance comes from toxicology work showing it just doesn’t pose a problem at low concentrations. But turn it up too far and that burnt, chemical aftertaste creeps in.
I once worked alongside a snack manufacturer experimenting with pyrazines to build flavor in a new peanut butter chip. One enthusiastic formulator doubled the amount, pushing it to 15 ppm. The lab filled with a scent closer to plastic than peanuts. Finished chips barely made it into the sample bowls before testers were shaking their heads. Everyone at the table learned fast — less is definitely more.
Consumers spot artificial notes quicker than you might think. A few extra micrograms can swing a blend from “authentic roasted” to “something’s off.” They don’t have to know the science to taste when the balance is wrong.
Though pyrazines don’t pose major health risks at typical levels, overuse can still cause headaches and digestive complaints. Repeated exposure above 15 ppm isn’t well studied, so sticking to proven, tested use is the way to go. Long-term, staying below the threshold helps keep food innovations safe — it also protects company reputations and keeps regulatory bodies off their backs.
If you’re dialing in your usage, consider starting around 1 ppm and climbing only as needed, testing in finished products alongside professional panels. Don’t “eyeball” it or rely just on aroma straight from the bottle — flavors intensify during cooking or baking. Keep records of what works, and build off those successes rather than risking over-flavoring and expensive recalls.
It’s tempting to chase a bolder note, especially with so many artificial flavors chasing “real” taste. Fact is, restraint works better. Makers using pyrazines should rely on both scientific guidelines and old-fashioned taste testing — those two together save money, time, and a lot of headaches down the line.
2-Butyl-3-Methyl Pyrazine carries an aroma that packs a punch, often finding its spot in flavoring products and fragrances. This stuff isn’t some mild kitchen spice – it’s used in micro-quantities, and even tiny leaks or spills can turn a workspace into a pretty strange-smelling place. The structure of this chemical makes it sensitive to its surroundings, so a careless approach won’t do.
There’s little value in buying top-grade material, then leaving it in an open jar or plastic bag. Glass bottles with tight seals, or high-quality HDPE containers, stand up best. Screw caps with liners keep air and moisture out, protecting the integrity of the pyrazine. This step saves headaches down the road – no one wants to trace a funky off-note in finished product back to a careless storage choice. Stainless steel drums sealed with gaskets also hold up well for larger batches. Polyethylene containers work as a budget pick, but even the best plastic can let through vapors over long periods, and flavors sometimes leach into the walls.
This isn’t about treating chemicals with unnecessary fuss. Heat speeds up chemical reactions, and light breaks down sensitive bonds. I've seen firsthand how samples stored on a sunny shelf that "should be okay for a few weeks" turn yellow-brown and smell a little off. Cool, dry, and out of the sun is the way to go. If a fridge isn’t an option, a closet or basement stays cool enough, as long as moisture stays low and no food’s nearby. Avoid freezing unless the supplier recommends it, since repeated freezing and thawing can change texture or flavor. Keep away from heaters and windows to reduce the chance of unpredictable shifts in quality.
Leave the cap off, and overnight, a film might form, or the aroma will fade. Pyrazines pick up moisture and odors from the air almost like a sponge. Even if a lab looks clean, air carries particles nobody sees but everybody smells eventually. Silica gel packets in the storage space pull a lot of moisture out of the air, which buys more time and saves product. A desiccator cabinet works even better but costs more.
Labels get overlooked. Grab a permanent marker and write the name, date, and lot number on every new container. Having relied before on mystery bottles, I can say that skipping this step leads to guessing, wasted material, and costly retesting. Anyone joining the team, or even your future self, will thank you later.
2-Butyl-3-Methyl Pyrazine doesn’t pose serious risks in tiny food-level amounts, but bigger accidents can leave lasting odors or cause headaches. Spill even a few drops, and clean-up takes the right gloves and good ventilation. Store absorbent material nearby. Keep storage areas separate from food and personal items. A whiff lingers, and once utensils or worktops pick it up, scrubbing sometimes just spreads the odor further.
Take the time to prepare storage so you don’t throw away half-used material later. Rotate stock the same way grocers do – use the oldest batches first. Write expiry dates where they’re easy to see. If large-scale usage is likely, invest up front in a chemical cabinet with controlled temperature. This cuts down on accidental losses and can even lower insurance costs for professional kitchens and labs.
Storing 2-Butyl-3-Methyl Pyrazine doesn’t call for a laboratory full of expensive equipment, but sloppiness adds up. The attention paid today saves resources and preserves the punchy, desirable aroma that makes this compound valuable in the first place.
| Names | |
| Preferred IUPAC name | 3-methyl-2-butylpyrazine |
| Other names |
2-butyl-3-methylpyrazine Butyl methyl pyrazine Butylmethylpyrazine 3-Methyl-2-butylpyrazine |
| Pronunciation | /tuː-ˈbjuːtɪl-ˈθriː-ˈmɛθɪl paɪˈræziːn/ |
| Identifiers | |
| CAS Number | 5521-55-1 |
| Beilstein Reference | 136700 |
| ChEBI | CHEBI:141500 |
| ChEMBL | CHEMBL515463 |
| ChemSpider | 167366 |
| DrugBank | DB03844 |
| ECHA InfoCard | 14b33e18-4f70-4488-a9b0-a3ac3a8c280a |
| EC Number | 256-923-7 |
| Gmelin Reference | 108031 |
| KEGG | C16268 |
| MeSH | D018469 |
| PubChem CID | 12306782 |
| RTECS number | UJ5959400 |
| UNII | QK7B52222J |
| UN number | UN3271 |
| Properties | |
| Chemical formula | C9H14N2 |
| Molar mass | 178.270 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | nutty, roasted, popcorn, cocoa |
| Density | 0.967 g/cm3 |
| Solubility in water | Insoluble |
| log P | 1.9 |
| Vapor pressure | 0.0144 mmHg at 25°C |
| Acidity (pKa) | 13.7 |
| Basicity (pKb) | 1.68 |
| Magnetic susceptibility (χ) | -75.0e-6 cm³/mol |
| Refractive index (nD) | 1.471 |
| Viscosity | Liquid |
| Dipole moment | 2.51 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 395.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | 165.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4148 kJ/mol |
| Pharmacology | |
| ATC code | '' |
| Hazards | |
| GHS labelling | GHS07, Warning, H315, H319, H335 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | P261, P271, P273, P280, P302+P352, P304+P340, P305+P351+P338, P312, P332+P313, P337+P313, P362+P364 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 99°C |
| Autoignition temperature | 370 °C |
| Lethal dose or concentration | LD50 (oral, rat) > 2000 mg/kg |
| LD50 (median dose) | LD50 (median dose): >5,000 mg/kg (rat, oral) |
| NIOSH | BX9510000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 2-Butyl-3-Methyl Pyrazine is not established. |
| IDLH (Immediate danger) | Not Listed |
| Related compounds | |
| Related compounds |
2-Isobutyl-3-methylpyrazine 2-Ethyl-3-methylpyrazine 2-Methylpyrazine 2,3-Dimethylpyrazine 2-Butylpyrazine 3-Methylpyrazine 2-Phenyl-3-methylpyrazine |