Chemists first explored the bromination of thiophene in the late nineteenth century. The breakthrough came as laboratories began to appreciate the subtle reactivity of heterocyclic aromatic systems. In those days, thiophene and its derivatives barely got a mention outside dye and dye-intermediate circles. Yet, introducing bromine to the thiophene ring sparked fresh curiosity; researchers soon realized that 2-bromothiophene could serve as a versatile building block. Over time, it became clear that this simple brominated thiophene carried more weight in the organic chemistry toolkit than its modest fame suggested, especially once palladium-catalyzed couplings became mainstream. The story of 2-bromothiophene isn’t only about industry: it’s also a story about how small molecules can change the direction of synthetic research and influence the way chemists think about constructing complex targets.
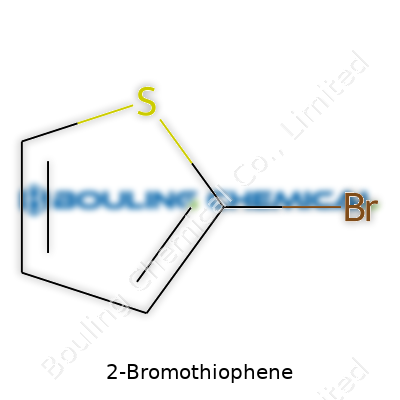
2-Bromothiophene offers a five-membered sulfur-containing ring with a single bromine atom at the second position. Its structure gives it unique reactivity for cross-coupling reactions, especially those needed for pharmaceutical synthesis and material science. Researchers, especially in drug discovery labs, use this compound to build larger and more complex molecules. Manufacturers typically supply it in clear or slightly yellowish liquid form, packed in sealed amber bottles that help shield it from light, which could otherwise degrade the compound. Labs and factories order it in quantities ranging from grams for research up to drums for industrial projects—always mindful of purity, since off-spec batches could derail sensitive reactions.
With a molecular formula of C4H3BrS, 2-bromothiophene presents itself as a flammable liquid with a sharp, characteristic smell reminiscent of halogenated aromatics. It boils at around 146–148 °C, melts at -23 °C, and weighs in at about 179.04 g/mol. Thanks to its modest polarity, it doesn’t dissolve well in water, but gives good solubility in organic solvents like ether or chloroform. Chemists notice its deep color under UV light, and some can spot it by scent alone. Once handled, it leaves a faint residue, not sticky, but persistent—a minor annoyance that reminds lab workers to glove up. The presence of both sulfur and bromine in its backbone gives it a special role in selective substitutions, arylations, and other transformations best performed under controlled lab conditions.
Manufacturers typically ship 2-bromothiophene labeled with its CAS number (1003-09-4), UN hazard classification (UN 2810, toxic liquid, organic, n.o.s.), and percent purity, which can reach up to 99%. Certificates of Analysis detail trace impurities, water content, and residual solvents. Both lab-scale and industrial buyers scrutinize these documents, knowing that catalytic performance and product safety ride on tiny differences in specification. Extra labels spell out storage restrictions—“keep tightly closed, protect from heat and moisture”—and safety phrases, such as R20 (harmful by inhalation) or S36/37 (wear suitable protective clothing and gloves). The best suppliers commit to transparent traceability and shipping practices, knowing that regulatory compliance matters just as much as chemical purity.
Chemists prepare 2-bromothiophene from thiophene itself by treating it with bromine or bromine derivatives, often under mild conditions with solvents like acetic acid. Some prefer using NBS (N-bromosuccinimide) for cleaner reactions and better selectivity. Temperature control proves crucial: too hot, and polybromination takes over; too cold and yields drop. Extraction with aromatic hydrocarbons and then vacuum distillation usually follows. The preparation isn’t particularly glamorous, but it highlights the care needed to tame reactive halogens and the importance of process control to keep batches consistent—a big concern for pharmaceutical manufacturers who cannot tolerate batch-to-batch drift.
2-Bromothiophene opens doors to a range of synthetic strategies. Its bromine atom invites Suzuki, Stille, and Heck cross-couplings, enabling the assembly of biaryl and polyheterocyclic compounds. Almost every advanced undergraduate or professional chemist has run a Suzuki coupling with 2-bromothiophene at least once—it's practically a rite of passage. Besides palladium-catalyzed reactions, nucleophilic substitutions and metal-halogen exchange reactions further expand its chemistry. Organolithium and Grignard reagents react at the bromo site, forming key intermediates that lead to more decorated thiophenes. At each step, the reaction conditions steer the reactivity and selectivity, demanding real skill and attention—sometimes a small adjustment in base or solvent shifts the product outcome.
The chemical world knows 2-bromothiophene under several aliases: it turns up as Thiophene, 2-bromo-; 2-Thiophenebromide; and sometimes as 2-BT or simply Br-thiophene. Some catalogs spell it as 2-bromothiofene. Patent literature throws more names into the mix for regulatory reasons, but those who work with the compound recognize it at a glance, regardless of name. Having multiple synonyms sometimes complicates digital searches or procurement, so cross-checking CAS numbers remains standard practice among procurement teams and lab managers.
Strict laboratory discipline surrounds the handling of 2-bromothiophene. Direct exposure to vapors or liquid causes irritation to the eyes and skin; inhalation risks shouldn’t be ignored. Fume hoods, goggles, butyl gloves, and flame-resistant lab coats become routine once the bottles come out. The compound's flammability and moderate volatility require careful solvent choice and storage in cool, ventilated spaces. Spills, though rare, demand prompt action—absorbent pads and dedicated waste bins are always on standby. For transport, international rules classify it as a hazardous substance, so shipping teams follow strict paperwork and packaging codes. Training matters: not all new chemists get this right without mentorship and oversight by experienced hands.
Pharmaceutical companies look to 2-bromothiophene as a springboard for new drugs, especially within anti-cancer and CNS research programs. It plays a role in synthesizing building blocks for antipsychotics and anti-inflammatory candidates. In materials science, the compound aids construction of conjugated polymers in organic electronics, photovoltaic cells, and OLEDs, where tailored heterocycles drive performance improvements. Agrochemical manufacturers use it to develop herbicide active ingredients or fungicides, tapping its chemical versatility. Many research universities rely on this molecule for chemistry demonstrations and student labs, as its robust chemistry lets students experiment without prohibitive costs. Its presence across these fields underscores its practical value beyond the obvious textbook reactions.
Newer studies tweak the reactivity of 2-bromothiophene by changing catalysts or introducing enabling ligands, pushing the boundaries for more selective C-H activations and eco-friendly transformations. Research groups invest resources to optimize scaling up, waste minimization, and greener halogen sources. Some teams work on tethering the thiophene core to bioactive moieties, hoping to uncover next-generation pharmaceuticals. In recent years, interest has also grown around computational modeling, where researchers use predictive tools to map out reaction pathways and design new derivatives before weighing out a single gram of starting material. Many pharmaceutical and electronics companies have ongoing programs built on this small but reactive unit.
Toxicity studies reveal that 2-bromothiophene can irritate skin, mucous membranes, and the respiratory tract. Prolonged exposure may cause more severe symptoms—headaches, nausea, or dizziness are not uncommon in poorly ventilated workspaces. Acute toxicity rests in the moderate range, but caution still rules every protocol. Chronic toxicity, mutagenicity, and long-term environmental effects currently attract more examination, especially in regions tightening chemical regulations. Scientists test degradation pathways, breakdown rates, and bioaccumulation in soil or water. So far, it tends to break down relatively quickly under sunlight, but formation of persistent brominated byproducts sparks ongoing concern and mandates proper disposal.
Looking ahead, the future for 2-bromothiophene lies in more sustainable chemistry. Researchers experiment with direct C-H functionalization, aiming to skip halogenation steps altogether and reduce hazardous byproducts. Material scientists see potential in advanced polymers, where finely tuned electronic properties rely on smart substitution strategies. Pharmaceutical candidates built off the thiophene core keep showing up in trials, driven by the compound’s proven versatility. AI-driven reaction prediction tools now get into the game, speeding up discovery and helping chemists avoid dead-end syntheses. Regulatory shifts will likely affect how companies handle, store, and dispose of 2-bromothiophene, but for now, its unique blend of reactivity and accessibility keeps it in high demand across research and industry.
If you walk into any chemistry lab and ask about 2-Bromothiophene, somebody will probably jot down “C4H3BrS” on the board. That’s the chemical formula, straightforward as it gets. You’ve got four carbons, three hydrogens, one bromine atom, and a sulfur atom sitting together in a five-membered ring. For those of us who have spent hours in the lab, recognizing the structure is more than memorization—it’s about understanding what those atoms mean in the larger context of chemistry and industry.
Chemistry folks know these formulas are more than just nerd trivia. I remember back in my college research days, small tweaks to a molecule, like swapping hydrogen for bromine, opened doors. 2-Bromothiophene holds a steady place in pharmaceuticals, agrochemicals, and advanced materials. You see, bromine sitting on the second carbon changes how this compound reacts with others. Anyone making specialty polymers or working in drug discovery has probably crossed paths with it. The reason is simple: the bromine atom acts like a handle for further reactions—it grabs attention in cross-coupling chemistry. That is the kind of transformation central to building bigger, more complex molecules.
It isn’t all sunshine and breakthroughs, though. Every chemist knows you can’t just focus on molecular formulas and ignore real-life risks. 2-Bromothiophene can be a health hazard: irritates the eyes, skin, and respiratory tract, and handling it demands respect for proper lab practices. I’ve seen careless mistakes with chemicals, and the lesson always kicks in fast—good ventilation and gloves aren’t suggestions, they’re essential. From another angle, brominated organics have a murky track record with the environment. Some linger longer than you’d expect and cause trouble in aquatic ecosystems. Stuff like this shows that chemistry never happens in a vacuum. Responsible handling and smart wastewater treatment come up in every serious discussion, especially when scaling up production.
Getting the formula right—C4H3BrS for 2-Bromothiophene—might seem as trivial as spelling your own name. Pass over it in a hurry and you risk mixing up compounds or screwing up a synthesis. I’ve dealt with mislabeled samples; the chaos that follows wastes time, money, and energy. Mistakes at the formula level can snowball into delays or failed batches. In my experience, double-checking these little details saves a lot of headaches. Teaching students or new lab mates, the mantra stays the same: treat every formula like it’s your home address. That mindset pays off, every single time.
Tools and technology help with accuracy and safety. Automated databases and smarter labeling software have cut down on mix-ups. Green chemistry pushes everyone to think about alternatives that are both effective and less troubling for the environment. Some groups are reworking synthetic routes to sidestep the need for brominated precursors. A few years ago, I’d mostly see talk on sustainability in conferences, now I see real changes on the bench. Chemistry keeps moving—sometimes it just takes a nudge from a simple string of letters, like C4H3BrS, to remind us why we keep striving for better solutions.
2-Bromothiophene doesn’t turn many heads outside of a chemistry lab. Thin, light yellow, kind of pungent—by itself, it’s not flashy. But this molecule matters, especially in the bigger picture of modern science and medicine.
Take a look at pharmaceuticals. A lot of medicine development turns to building blocks like 2-Bromothiophene. Think about antibiotics, pain relievers, or treatments for allergies—many of these rely on “substituted thiophenes.” To get there, chemists lean on this handy molecule as a starting point or an anchor in molecular synthesis.
I once visited a friend doing postdoc work in medicinal chemistry. His benchtop held flasks full of odd-smelling liquids, including one labeled “2-BrTh.” When he explained what he was up to—crafting new anti-inflammatory candidates—I got a sense of how foundational building blocks such as 2-Bromothiophene shape the future of medicine. He would make tiny tweaks to the thiophene ring, hunting for a new function or less toxic side effect.
According to a 2022 report in the journal “Bioorganic & Medicinal Chemistry,” more than a dozen drugs use thiophene rings at their core. For chemists, 2-Bromothiophene provides a shortcut into those complex structures. The bromine atom acts like a handle, letting researchers swap out pieces with relative ease.
Move outside the pharmacy, chemists in electronics labs see 2-Bromothiophene as a gateway to advanced materials. Over the last couple decades, scientists started making plastics that carry electricity—unlike the stuff coating wires. The backbone of a lot of these “conducting polymers” includes thiophene rings. In the early days, polythiophene helped build simple solar cells and flexible displays.
The bromine tag in 2-Bromothiophene lets researchers bolt these rings together in chains or branches. They can fine-tune the colors, durability, and conductivity of the final material. OLED frames, organic solar panels, flexible screens—these all trace back to organic synthesis, and 2-Bromothiophene often plays a supporting role.
Handling 2-Bromothiophene isn’t always easy. The liquid can irritate skin and lungs, so proper gear is a must. Transport involves strict packaging, and not everyone has the facilities to store or dispose of leftovers safely. There’s increasing pressure in the chemical industry to cut down on hazardous waste, something that I’ve watched gain momentum at industry conferences—no one wants to keep paying fines or put workers at risk.
One potential answer: greener alternatives to the old bromination processes. Some research teams have swapped in less toxic reagents; others recycle their reaction waste. Labs are moving toward microreactors, where smaller batches mean fewer accidents and less waste. Scale matters, so these types of processes could shrink the environmental footprint of industrial manufacturing. Many in the field want to keep synthesizing the molecules the world needs, but do so more responsibly.
The world would look different without chemicals like 2-Bromothiophene. Whether it’s scientists at the bench tweaking molecules for new drugs or engineers assembling the next wave of electronics, this compound often sits quietly in the background. But understanding those roles doesn’t just push chemistry forward—it shapes medicine, energy, and sustainability efforts. Paying attention to these small ingredients might just open the door to real change in both our tech and our health.
Watching a clear liquid bubble in a flask has a bit of magic to it – but in the chemistry lab, it's all about knowing what those bubbles mean. In school, I spent hours poring over tables of boiling points, and every compound brought its own quirks. 2-Bromothiophene stands out in my mind. Its boiling point, sitting around 192–194°C, always caught some attention among classmates, especially when compared to its relatives. That detail seemed unremarkable until I actually had to distill it for a summer research project. Suddenly, every degree mattered.
A boiling point is more than a number to memorize. In real life, you need it for safe handling and planning reactions. If a compound evaporates before your reaction finishes, that’s wasted time, wasted chemicals, and sometimes fumes that clear out the lab. 2-Bromothiophene’s boiling range—just under 200°C—puts it in a spot that’s reasonably stable for work but still easy to vaporize compared to some clunky, heavy molecules.
The structure of 2-Bromothiophene brings together a sulfur-containing ring and a bromine atom, creating a molecule that pops up in pharmaceutical development and advanced materials. Its boiling point isn’t random. That ring keeps it from shaking apart too fast, and the bromine tugs it toward a higher temperature compared to plain thiophene, which boils at just 84°C.
Lab work isn’t forgiving if you ignore physical properties. That lesson hit hard during my first attempt at purifying 2-Bromothiophene. I used the wrong setup, underestimating how sharp the vapor can be. The solution boiled over faster than I expected, and the smell—a mix of sweet and burnt rubber—clung to the room for hours.
After that mess, I read up. Ventilation isn’t optional. Open flames don’t go near brominated solvents, and distilling above 190°C takes glassware that can handle the heat without cracking. Lab manuals are full of warnings, but nothing replaces seeing a reaction run away in real time. Misjudging a boiling point can make you the reason for an unscheduled break while fume hoods cycle the air.
Industry leans on boiling points for separating chemicals and scaling up synthesis. Distillation processes get expensive fast if temperatures soar, so 2-Bromothiophene’s manageable boiling point can make production smoother. Pharmaceutical companies use it for building blocks in drug synthesis, and that tiny temperature detail determines whether a reaction setup costs hundreds or thousands to run.
A new chemist, armed with facts instead of guesses, can avoid most rookie mistakes. Double-checking the boiling point before turning on the hot plate makes work safer and more predictable. Organizing data tables, keeping clear labels on bottles, and respecting ventilation—these habits eventually save time, money, and sometimes your own health.
Boring as tables of boiling points may seem, ignoring them quickly turns a smooth day in the lab into a cleanup operation. Next time I see 2-Bromothiophene on a reagent shelf, that 192–194°C sticks in my head, not because it’s an exam answer, but because experience taught me what can go wrong if you don’t respect the numbers.
Nobody likes a spill in the lab, especially with a chemical like 2-Bromothiophene on hand. Every chemist, no matter how seasoned, has that story about catching a whiff of something pungent and realizing a cap wasn’t closed. 2-Bromothiophene, with its low boiling point and strong odor, always serves as a reminder to prioritize good habits over shortcuts.
Sitting on the shelf, 2-Bromothiophene looks pretty harmless in a bottle, but it hardly acts like it. The compound is flammable and can irritate the eyes, skin, and respiratory tract. Its volatility means the bottle, if left loose or exposed, will eventually fill the room with fumes. Chemical storage isn’t just about ‘putting it away’; it’s making sure the next person walking in won’t get hit with a dangerous vapor cloud.
Out of habit, I’ve always double-checked bottles before I leave for the day. It only takes one slip to cause trouble. On one busy afternoon, a bottle of 2-Bromothiophene left open ruined hours of work. Beyond the irritation, it posed a hazard to everyone nearby. Simple routines—like storing such chemicals in tightly sealed glass bottles and keeping them cool—save more time and resources in the long run.
2-Bromothiophene thrives in a cool, dry place, away from sunlight and sources of ignition. I’ve witnessed what happens when storage gets sloppy—labels fade, rubber stoppers degrade, and the potential for an accident shoots up. Some folks try to cut corners, stuffing bottles near heat-producing equipment or ignoring humidity. Those practices result in glass corrosion and chemical breakdown, something nobody wants to deal with.
Storing this compound near oxidizing agents or acids invites problems. Those substances have a way of finding each other and reacting when least expected. Proper shelving—well-ventilated, segregated, and labeled—helps avoid surprise reactions and unnecessary cleanups. Good ventilation isn’t just about comfort; it prevents vapor concentration and buildup, reducing risk every single day.
Fire-proof cabinets and chemical fume hoods aren’t just recommendations—they are essentials. Most labs that value safety make them part of standard practice. Flammable chemicals call for metal cabinets or flame-resistant storage, with absorbent materials ready nearby in case of leaks. Lab coats and gloves come before stylish outfits, and safety goggles never go out of fashion in this line of work.
Lab teams use clear inventory records, check expiration dates early, and order only as much as needed. Some folks label containers with bold warnings or color codes; others track movement with logbooks. Whether you rely on tradition or automation, clear policies and routine audits save lives.
Protecting people, property, and research means storing 2-Bromothiophene with respect. Keeping it cool, dry, and isolated stands out as the single best habit. I’ve learned that setting a good example saves headaches and hospital visits down the road. Small choices in storage add up quickly, turning a potentially risky bottle into just another safe part of science.
Most people walking into a chemistry lab for the first time probably don’t think much about the bottles lining the shelves. They all look pretty similar—clear glass, printed hazard labels, and some unpronounceable names. 2-Bromothiophene, though, deserves a little more respect than that. It’s not famous like the acids or bases you meet in high school, but it creeps into many synthetic labs, especially for those working on pharmaceuticals, dyes, or new materials.
So, what’s the danger? 2-Bromothiophene doesn’t show up flashing neon warnings, but don’t let that fool you. The liquid carries a sharp, unpleasant odor, hinting at its volatility. If you spill a bit, the smell hits fast and lingers. It evaporates more easily than water. Your standard blue gloves don’t offer much comfort—splashes sting the skin, and that burning sensation can last a while. Even on a mild day, a bit of the chemical vapor gets into your eyes or lungs and the irritation starts quickly. I’ve seen experienced researchers cough and blink rapidly after an accidental whiff—the stuff slides into your system without much warning.
Short-term irritation is annoying, but the real risks show up in chemical safety sheets. 2-Bromothiophene can mess with your liver and nervous system at higher exposures. Researchers in Tokyo once flagged chronic exposure as an underestimated risk, especially in poorly ventilated labs. Contact dermatitis builds over time, not in a single day, but in layers. Inhalation of vapors or accidental ingestion gives you far more trouble—nausea, headache, and, over months or years, potentially worse. Some structural relatives of thiophenes carry cancer concerns as well, though data on 2-Bromothiophene itself is thin. I always find it smart to err on the side of caution with halogenated organics, even if the jury’s still out.
Carelessness doesn’t fit well in a lab, but plenty of chemists get cavalier with organics like these. I remember a time a bottle broke near a hotplate—nasty fumes clouded the room within seconds. The fume hood sat unused at the next bench over, probably because someone wanted to save time. That mistake led to headaches for the whole team, followed by a mad dash to improve ventilation and rethink our habits.
Safe handling isn’t rocket science, but you can’t cut corners. Good ventilation comes first—every procedure with 2-Bromothiophene should happen in a working fume hood. Chemical splash goggles beat regular safety glasses. Gloves make a difference, but ordinary nitrile doesn’t cut it; thicker butyl or laminated gloves stop more of these molecules from sneaking through. Spills call for fast, organized cleanup, with absorbent pads rated for organics. We kept sand and waste bags handy for fast disposal.
Label bottles clearly, and store them away from heat sources. Even the sturdiest glass bottles deserve a secondary container when moving between rooms. Training new lab members to respect every unfamiliar reagent proves essential. No need to scare off rookies, but a little wariness keeps everyone out of the campus health clinic.
Most labs rely on checklists and reminders posted right at the bench. Audits help, but nothing beats daily habits. People shouldn’t learn the hard way that 2-Bromothiophene means business. Once you’ve watched someone flush out their eyes or explain a spill to environmental health, you start to appreciate the routines. A bit more planning, better gear, and steady habits take the pressure off in the long run.
| Names | |
| Preferred IUPAC name | 2-Bromothiophene |
| Other names |
2-Bromothiophen Thiophene, 2-bromo- 2-Thienyl bromide |
| Pronunciation | /ˈtuː broʊmoʊ θaɪ.oʊˌfiːn/ |
| Identifiers | |
| CAS Number | 1003-09-4 |
| Beilstein Reference | 1209223 |
| ChEBI | CHEBI:141527 |
| ChEMBL | CHEMBL515181 |
| ChemSpider | 63277 |
| DrugBank | DB08385 |
| ECHA InfoCard | 03a8155780-a682-49d2-b6c3-dae3575ad5b4 |
| EC Number | 209-800-6 |
| Gmelin Reference | 7897 |
| KEGG | C06724 |
| MeSH | D017896 |
| PubChem CID | 69110 |
| RTECS number | XM8925000 |
| UNII | T9VAN55DVK |
| UN number | UN2345 |
| CompTox Dashboard (EPA) | DTXSID1041818 |
| Properties | |
| Chemical formula | C4H3BrS |
| Molar mass | 191.07 g/mol |
| Appearance | Colorless to yellow liquid |
| Odor | Aromatic |
| Density | 1.654 g/mL at 25 °C |
| Solubility in water | slightly soluble |
| log P | 1.98 |
| Vapor pressure | 0.7 mmHg (20°C) |
| Acidity (pKa) | 5.17 |
| Basicity (pKb) | -7.45 |
| Magnetic susceptibility (χ) | -60.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.595 |
| Viscosity | 2.464 cP (20°C) |
| Dipole moment | 1.95 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 339.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | 28.2 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -3207 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | InChI=1S/C4H3BrS/c5-4-2-1-3-6-4/h1-3H |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P210, P261, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 2-2-0 |
| Flash point | 54 °C |
| Autoignition temperature | 572 °F (300 °C) |
| Explosive limits | Explosive limits: 1.4–9.3% |
| Lethal dose or concentration | LD50 oral rat 1900 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral, rat: 1400 mg/kg |
| NIOSH | PA9475000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 1 g, 5 g, 25 g |
| IDLH (Immediate danger) | Unknown |
| Related compounds | |
| Related compounds |
Thiophene 2-Iodothiophene 2-Chlorothiophene 3-Bromothiophene 2-Bromofuran |