Digging into the history of thiophene chemistry feels like reading a manual written with real grit—chemists constantly pushing to find the right twist on heterocyclic compounds. By the time synthetic chemists started attaching both a bromine and a formyl group to the five-membered thiophene ring, the need wasn’t just academic. Researchers in the late 20th century wanted specialty building blocks for pharmaceuticals and electronic materials, and simple bromothiophene no longer cut it. Early syntheses struggled with selectivity, and yields often fell short. Persistent tinkering and advances in handling organobromine compounds opened doors to more reliable ways to get hold of this molecule, letting it step out from the shadow of its simpler relatives.
2-Bromo-5-Formylthiophene looks like a modest player—transparent yellow liquid or sometimes pale solid, depending on storage and temperature. Under the surface, this compound has a knack for enabling reactions that plain thiophene never could. Researchers reach for it when they want a handle for cross-coupling or elegant substitutions. I’ve seen organic synthesis teams value it for its reliability, the kind where you don’t wonder if your starting material will throw a curveball. In practice, most appreciate its balance—reactive enough to be useful, but stable enough to keep on the shelf for more than a few frantic weeks.
Staying grounded in the concrete, 2-Bromo-5-Formylthiophene has a molecular weight around 205 g/mol. It boils in the range of 80-82 °C under reduced pressure, and its melting point sits near room temperature—nothing dramatic, but still worth watching during purification. The molecule’s aromatic ring, lined with sulfur and dotted with bromine and an aldehyde group, brings a bitter-almond odor reminiscent of other thiophene aldehydes. It doesn’t cheerfully mix with water, preferring organic solvents such as dichloromethane and ethyl acetate. That hydrophobic quality both simplifies and complicates handling, especially during workup or column chromatography.
Every bottle or ampoule should show its CAS number (122372-35-4), molecular formula (C5H3BrOS), purity (usually over 97%), and batch number for traceability. Most labs want HPLC or GC data for reassurance. The yellowish tint signals a clean product, since browning can point to degradation or the presence of tricky sulfur byproducts. The density, stored close to 1.7 g/cm³ at ambient, signals a hefty halogen presence. Material Safety Data Sheets flag storage in cool, dry areas, away from direct sunlight—any heat or stray moisture risks slow decomposition and makes the glassware sticky.
Reliable synthesis often starts from 2-bromothiophene. Practitioners generally swing for a formylation reaction, with a Vilsmeier-Haack setup delivering a solid route. This means treating 2-bromothiophene with DMF and POCl3, which plants the formyl group at the 5-position with decent selectivity. Workups sometimes make you sweat during the separation of regioisomers, and fine-tuning the ratio of reagents can mean the difference between a decent yield and a frustrating repeat. Minor routes use lithiation of 5-bromothiophene and subsequent reaction with DMF, but sensitivity to moisture requires extra caution—one careless move, and half the batch may end up wasted.
The value of 2-Bromo-5-Formylthiophene rests in its dual functional groups. The bromine atom often serves as a launchpad for Suzuki and Stille couplings, giving researchers access to all kinds of substituted thiophenes and extended π-systems for electronics. That formyl group opens doors for transformations—condensation to form imines, oxidation to carboxylic acids, reduction to alcohols, or participation in Knoevenagel reactions. In the real world, chemists run these reactions to introduce complexity simply, saving multi-step procedures and their frustrations. Even small modifications to the thiophene core have outsized effects on the resulting material’s optical and electronic features, a fact that drives ongoing innovation in organic semiconductors and light-absorbing compounds.
Depending on the supplier or context, you might call this compound 2-Bromo-5-thiophenecarboxaldehyde, BFT, or 2-Bromo-5-formylthiophene. The nuances of name selection often reflect the habits of a specific research group or a synthetic chemist’s loyalties. Synonyms show up in technical sheets and patents, so keeping them straight saves time, especially during literature searches or when tracking down regulatory documents.
Direct experience in the lab leaves no illusions: 2-Bromo-5-Formylthiophene is not benign. It acts as an irritant to skin, eyes, and mucous membranes. Many MSDS sources stress glove and goggle use, and fume hoods stand as non-negotiable. Before disposal, neutralize its residues to avoid local environmental impact. Disposal through incineration suited for halogenated organic compounds keeps liabilities at bay. Spills, even minor ones, require careful wipe-up with inert absorbents. Once, a mishap involving a leaky septum led to a lasting odor in the lab, driving home just how stubborn thiophenes can be.
Academia and industry both turn to this molecule for its applications in making conjugated polymers, organic conductors, and pharmaceuticals. In organic electronics, the compound lets teams design new thiophene-based building blocks with tuned photophysical properties. Medicinal chemists have tried sliding substituted thiophenes into candidate drug libraries, looking for metabolic stability and bioactivity that other scaffolds can’t match. In agrochemical research, thienyl derivatives keep cropping up as potential herbicides and fungicides. These applications work because the molecule’s functionalities can be dialed in with existing synthetic methods, allowing for real-world flexibility in material design.
Current R&D focuses on broadening the utility of 2-Bromo-5-Formylthiophene in the synthesis of advanced materials. Teams studying organic field-effect transistors report that small tweaks in thiophene structure can impact charge mobility and stability. In solar cell research, pairing these molecules with other donor-acceptor systems improves absorption and device lifetimes. Pharmaceutical groups explore its derivatives for antimicrobial and anti-inflammatory action, designing libraries with substitutions across the aromatic ring. Each discovery points to how far a single substitution pattern can stretch in the search for new function.
Thorough toxicity studies on 2-Bromo-5-Formylthiophene itself remain limited, but anecdotal evidence and similar compounds suggest caution. Brominated aromatics often show chronic toxicity or environmental persistence, and aldehydes tend toward cytotoxicity at higher doses. Recent reports flag oxidative stress and mild mutagenicity in cell culture assays for related thiophene derivatives. Those in charge of handling or disposal keep a close watch on exposure limits, and environmental safety committees frequently update guidelines for handling organosulfur and organobromine waste. Experience teaches the value of going beyond the legal minimum; biological effects sometimes emerge only after years of routine use.
Looking to the future, researchers will keep seeking better ways to synthesize, apply, and study derivatives of 2-Bromo-5-Formylthiophene. The drive for greener, more sustainable chemistry will shape handler practices and synthetic routes, pushing for mild, low-waste processes. Innovations in catalysis could ease bottlenecks and open new reactivity pathways unavailable today. Applications in electronics and photonics remain strong, with material scientists striving to harness every bit of structural novelty from thiophene chemistry. Improvements in computational chemistry may help predict toxicity and environmental impact far earlier in the R&D cycle, cutting down on unintended consequences. I expect the conversation around this molecule to balance real-world pragmatism—safety, scalability, flexibility—with the relentless curiosity that scientists bring to every corner of synthetic chemistry.
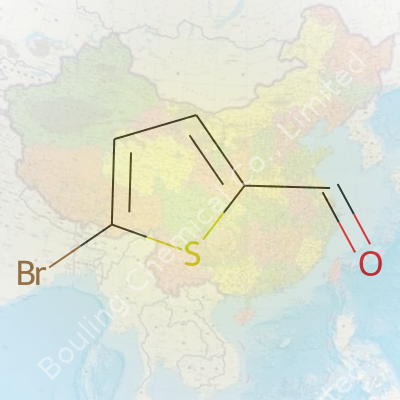
Step into any organic chemistry lab, and sooner or later you bump into something like 2-Bromo-5-Formylthiophene. For anyone who remembers scribbling chemical formulas on notepads, this one comes down to C5H3BrOS. That tells a story. Five carbons form the backbone, bonded to three hydrogens, a bromine atom, an oxygen, and a sulfur. The way these pieces fit together gives it a special place in the realm of aromatic aldehydes. Toss a carbon position here or there, and the behavior of the molecule changes completely.
Hit the books, check the tables: C5H3BrOS puts you at a molecular weight of 206.05 grams per mole. Weighing things out in the lab, accuracy is everything. A slip—just a few milligrams—and an entire reaction might fizzle. Back in chemistry class, weighing tiny crystals felt tedious. Out in the field, it means saving hours or even weeks by getting conditions right on one try. Nobody wants a partner grumbling over lost sample in the sink.
Once you realize the world rests on basic building blocks, interest in quirky molecules grows. 2-Bromo-5-Formylthiophene isn’t just another compound, either. Start digging into journals or patent filings, and you run across research in pharmaceuticals, OLED displays, and even solar cells. Each time, that formula and weight decide what happens next. In drug discovery, changing one atom can flip a molecule from useless to life-saving. You need the numbers before you walk into the lab.
Chemists get stuck when information about compounds—like formulas or weights—go missing or vary between sources. Every lab has a binder filled with post-it notes of corrections. I remember comparing supplier catalogs, trying to confirm if the formyl group was where it belonged or if the molecular weight factored in isotopic bromine distributions. Inaccurate data wastes time, and it isn't just a classroom headache. Real-world research depends on that foundation.
Another issue: safety. Bromine compounds often demand care. Wearing the right gloves and working under a hood become habits that stick with you. Employees in manufacturing environments sometimes receive little explanation. Having the chemical formula in hand, you start connecting the dots to Material Safety Data Sheets, spotting risks quickly rather than calling poison control for help later.
For students and new researchers, using a structure drawing tool—something like ChemDraw—lets you double-check formulas instantly. Setting up lab protocols that start with clear verification of reagents saves time and improves results. Professors who spend time explaining why formula accuracy matters often see fewer accidents in the lab. On a broader scale, open-access chemical databases offer fast, reliable information, keeping old mistakes from cycling back into experiment after experiment.
More clear labeling from suppliers and stricter quality checks in chemical inventories push research forward. I’ve yet to meet a chemist who enjoys running the same experiment twice because of a catalog misprint. Every clear answer—like the chemical formula and molecular weight of 2-Bromo-5-Formylthiophene—turns the fog of uncertainty into something like a map, making science just a little less intimidating for the next person facing down a fussy reaction.
Walk into any modern chemistry lab and you’re likely to spot shelves lined with jars of reagents that sound exotic, like 2-Bromo-5-Formylthiophene. Most people haven’t heard of it, but those working in organic synthesis know its value. Years ago, during a stint in a medicinal chemistry lab, I saw how much rides on having the right building blocks to put together novel molecules. Anyone who’s spent time doing multi-step synthesis can recall the frustration of being one reagent short of a breakthrough. 2-Bromo-5-Formylthiophene sits among those select chemicals able to turn a routine day at the bench into a turning point for a new drug or an advanced material.
A medical chemist looking for new treatments often needs to build a library of compounds—tweaking a structure just so, to find better activity or lower toxicity. 2-Bromo-5-Formylthiophene brings together a bromine atom and a formyl group on a thiophene ring. That combination lets scientists reach into a huge toolbox: the bromine acts as a gateway for Suzuki, Stille, or Heck couplings. The formyl group, small as it seems, lets researchers tack on new fragments using well-known reactions like the Wittig or Knoevenagel condensations.
Drug development takes patience, and sometimes it’s not only the big pharma giants pushing boundaries. In university labs, grad students mix and match different pieces, just hoping to hit on a compound with antiviral or anti-cancer effects. By making it easier to link up small rings and side-chains, 2-Bromo-5-Formylthiophene helps speed the journey from whiteboard sketch to possible medicine.
It’s not all about health care. Over the last decade, thiophenes found a new home in the world of organic electronics—think solar cells and OLED displays. Flexible, lightweight screens that roll up or bras that track heart rates all depend on improved organic semiconductors. Many of the compounds used as active layers in these devices start with a thiophene core. Add a bromine and a formyl group to the ring, and the chemist gets the perfect handle to snap on custom side chains. By changing the chain, they control how the molecule lines up on a surface, how it absorbs light, maybe even how long the device will last.
The demand for better, greener electronics has pushed more researchers to experiment with thiophene derivatives like this one. The routes built with 2-Bromo-5-Formylthiophene can help small startups make lightweight solar panels for off-grid homes or flexible circuits for the next generation of wearable gear.
Cost and safety sometimes hold back the more widespread use of chemicals like this. Anyone who’s spent time in a lab has probably run into trouble with tricky purification steps or hard-to-store intermediates. Tighter supply chains put new pressure on labs to find reliable, safe, and economical ways to use key reagents. Companies and academic labs could pool resources—maybe share new synthetic tricks or open up access to greener production methods. Smaller-scale researchers benefit from pre-made building blocks too, which lets them focus on solving real problems rather than wrestling with hard-to-source chemicals.
The bigger story here lies with collaboration: pooled data from different labs track how small changes to building blocks can spark smarter medicines or more reliable devices. Sharing insights and practical tips on handling and using 2-Bromo-5-Formylthiophene may do more for science than any one big discovery.
2-Bromo-5-Formylthiophene shows up in a lot of research labs. With a reputation for helping chemists build new molecules or develop targets for biotech, it’s not something you’d want spilling onto your hands or drifting into your lungs. I remember the first time I handled a similar thiophene—it taught me respect for the power that these little bottles can carry. Gloves, goggles, and a tight plan make all the difference.
Handling this chemical means using gloves that don’t give up under stress. Nitrile often works better than latex here. Forgetting eye protection isn’t an option, especially in labs with poor ventilation or shoddy hoods. As a rule, I never cracked a bottle open unless the fume hood was running and someone could spot me in case of trouble. Even the most seasoned folks can get caught off-guard by splashback or surprise fumes.
The phrase “cool, dry, and well-ventilated” gets repeated so often in safety training that it turns into background noise, but it truly matters. Safeguarding 2-Bromo-5-Formylthiophene isn’t about ticking boxes. Moisture in the air may change the composition over time, especially if containers sit open between uses. Sunlight’s even worse, speeding up those slow chemical changes you won’t notice until your experiment flops.
Every time I’ve transferred bottles into flame-proof, chemical-safe cabinets—even if it meant an extra walk across the building—there’s a payoff. No panicked runs to the sinks, less drama if someone accidentally bumps the wrong shelf, and peace of mind for everyone.
Too often, labs treat small spills as minor events. Less than a milliliter hits the bench, and someone wipes it up with a paper towel and keeps going. Cutting corners never pays off. Small leaks can mean breathing in vapors, causing throat irritation or headaches that linger all day. For me, any chemical spill—no matter the amount—meant gloves came off, surfaces got wiped with proper solvent, and waste went in a labeled container. Eyes on the bottle, not the clock.
Getting familiar with the MSDS isn’t busywork—it's critical survival reading. It tells you what to avoid mixing, what to do if skin contact happens, and the right cleanup routine. At one point, I saw someone pour a splash of water near a brominated bottle on a steel tray. The result: heat, a sharp smell, and a scramble for the vent. A little reading beforehand would’ve saved everyone the scare.
Bottling up reminder alarms, checklists, and shared habits does more than protect the researcher. It prevents ruined experiments, keeps the budget intact, and protects the planet by reducing waste. I’ve found group safety audits and “practice drills” before starting a new project get everyone in the mindset to respect every bottle as if it held something irreplaceable.
So each time you reach for 2-Bromo-5-Formylthiophene, remember: small habits—tight gloves, right goggles, secure containers—keep the work smooth and the stories boring, just the way chemical safety ought to be.
The world of specialty chemicals leans heavily on fine details. 2-Bromo-5-Formylthiophene isn’t just another reagent—it’s a compound with a pretty specific job, mostly in research, drug development, and some materials science circles. Purity matters here—not just for a tidy batch, but for reactions to actually go right. It’s common to see companies list purity above 97%, with 98% and even 99%+ available for labs where even a percent off can send a synthetic route sideways. I’ve run experiments where getting stuck with something under 98% meant side products and wasted time, so seeing a high number right on the label brings real relief.
Many suppliers—Cayman, TCI, Sigma-Aldrich, and Alfa Aesar—push these high-purity grades as standard. They provide a certificate of analysis or a datasheet with each batch. I’ve always checked those figures myself because buying on trust alone leads to nasty surprises. It’s not just the big brands, either. Producers in India and China now match these purity benchmarks, driven by researchers who won’t put up with odd results or impurities slowing publications and new drug screening.
It’s odd how much trouble packaging size can cause if you get it wrong. In the early days, I’d over-order a 25g bottle only to find half of it crusting in the back of a cabinet months later. Labs that run lots of trials between screening and scale-up want options. Here, 2-Bromo-5-Formylthiophene behaves like many fine chemicals. The most common size you’ll see sits at 1g per bottle—big enough for method development, small enough to work with safely and keep on hand. It’s not rare to spot 5g and 10g jars aimed at those who need to run multi-step reactions or prepare reference compounds for whole teams.
On those occasions I’ve talked with suppliers about scale-ups or pilot manufacturing projects, 25g and 100g bottles start to show up. Going to the 500g or kilo scale is possible if you’re working for a pharmaceutical or doing a big contract run, but usually these come as custom orders. Most small companies don’t hold on to huge quantities because the market just isn’t that big, and this avoids sitting on old stock.
Purity and packaging both speak to safety and shelf stability. Moisture and air eat away at delicate chemicals, so small glass bottles with septa or crimp tops show up often. Some suppliers claim light-resistant amber glass, but many labs transfer right into their own vials under nitrogen. I shot myself in the foot once letting a sensitive aldehyde sit out too long, and that lesson sticks: Store it right or risk starting all over.
High-purity 2-Bromo-5-Formylthiophene doesn’t come cheap. A researcher might pay $70 or more for a gram from a Western supplier, while some bulk offers from overseas get to $600 for 100g. Customs, hazardous shipping fees, and batch testing all play their parts. There’s an ongoing debate about lowering entry barriers for university labs and startups, but right now, cost reflects purity and reliability.
If there’s a push for improvement, it should focus on two things: Fast, cheap, reliable testing for purity—so researchers can double-check their stock— and more standardized small package sizes, to stop waste and boost accessibility. Clearly labeled, promptly shipped, with real data in hand, that’s the target. Anyone who’s lost a week chasing an impurity band in an NMR knows how badly real-world transparency matters.
2-Bromo-5-formylthiophene pops up quite often for chemists hunting for functionalized five-membered rings. The molecule gives you both a bromine atom and an aldehyde in its toolkit, letting you build all kinds of new compounds. Most synthetic work demands tough choices—finding the steps that strike a balance between purity, cost, safety, and plain reliability.
Most chemists reach for bromination on a preformed 5-formylthiophene scaffold. Starting from 5-formylthiophene, which already carries the tricky aldehyde, means you only need to introduce that bromine. Electrophilic bromination lands the halogen at the 2-position, thanks to the electron-withdrawing effect of the aldehyde at the 5-position. N-Bromosuccinimide (NBS) works as a solid pick—it's safer and easier to handle compared to straight molecular bromine, plus you get selectivity if you keep the temperature low and quench the reaction quickly.
From experience, some chemists struggle with overbromination or problems tied to side-reactions. Setting up the right ratio of NBS and running the reaction in an inert solvent like DMF or chloroform helps keep the unwanted junk down. It's easy enough to check on the crude product using TLC or HPLC, so you catch problems early instead of letting the reaction stew too long.
Sometimes you can take the longer road and start with 2-bromothiophene, then tack on the formyl group. The Vilsmeier–Haack formylation creates the aldehyde with decent efficiency. Using POCl₃ and DMF, the reaction adds the formyl at the less hindered 5-position. This approach saves time if you have bromo-based precursors on hand, and the byproducts are usually simple to separate out.
This method brings its own headaches. The reaction gets hot fast, and POCl₃ isn't the friendliest chemical in the lab. Good ventilation and gloves never go out of style, but here they're essential. Quenching the reaction carefully, followed by extraction with a mild base, usually helps tone down the harshness. More experienced hands often lean this direction if the scale calls for a sturdy, dependable result.
Once you have your 2-bromo-5-formylthiophene, the avenues open up. I've seen chemists use the bromine handle for Suzuki–Miyaura or Stille couplings, joining anything from aryl to alkyl groups. Palladium catalysis brings impressive selectivity and mild conditions. Careful control of base and temperature means the aldehyde stays untouched—a big plus since the formyl group can wander or disappear under harsh conditions.
Transforming the aldehyde can be just as satisfying. Reductive amination, for instance, lets you install amines in a single pot. Sodium borohydride and a primary amine send the compound down a useful synthetic fork, opening up all sorts of heterocyclic or pharmaceutical possibilities. The formyl function gives you easy access to imines, alcohols, or even further cyclizations—someone in medicinal chemistry never lacks for ideas at this stage.
The tough spots show up fast: competing side-reactions, reagents that oxidize or polymerize your target, or just sticky material that's tough to purify. Silica gel chromatography still handles most crude material, but using hexanes with a bit of ethyl acetate helps avoid loss of product. I found that keeping reaction times short and purifying immediately gives a higher yield and avoids product decomposition.
For anyone facing scalability, slow addition of reagents and jacketed glassware for temperature control make a difference. Working in small batches and building up to multigram scales keeps things in check and limits waste. Lab safety always stands front and center—not just for yourself, but everyone sharing bench space.
Seeing 2-bromo-5-formylthiophene go from raw reagents to a clean, crystalline product never gets old. The practical challenges reward anyone willing to keep a sharp eye on the details and an open mind toward procedural tweaks. Innovation shows up in real time—running pilot reactions, comparing solvent systems, switching up coupling partners. That’s the kind of tangible progress that keeps synthetic chemistry rooted in hands-on experimentation, not armchair theorizing.

| Names | |
| Preferred IUPAC name | 5-Bromothiophene-2-carbaldehyde |
| Other names |
5-Bromo-2-thiophenecarboxaldehyde 2-Bromo-5-thiophenealdehyde 2-Bromo-5-formyl-thiophene |
| Pronunciation | /tuː-ˈbroʊmoʊ-faɪv-fɔːrˈmɪl-θaɪˈoʊfin/ |
| Identifiers | |
| CAS Number | [16294-37-8] |
| 3D model (JSmol) | `JSmol.load("data:text/plain;base64,3dmodelfile/V30002-Bromo-5-Formylthiophene...")` |
| Beilstein Reference | 1367086 |
| ChEBI | CHEBI:189285 |
| ChEMBL | CHEMBL3936847 |
| ChemSpider | 23856992 |
| DrugBank | DB04126 |
| ECHA InfoCard | 05a160ad-3a84-41dd-8595-3acfc8dd158d |
| EC Number | 607-313-8 |
| Gmelin Reference | 1040956 |
| KEGG | C18529 |
| MeSH | D04702 |
| PubChem CID | 676360 |
| RTECS number | XT5950000 |
| UNII | UP9GX6E261 |
| UN number | UN3439 |
| Properties | |
| Chemical formula | C5H3BrOS |
| Molar mass | 204.08 g/mol |
| Appearance | Yellow to orange solid |
| Odor | pungent |
| Density | 1.789 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 1.9 |
| Vapor pressure | 1.4E-3 mmHg at 25°C |
| Acidity (pKa) | 20.01 |
| Basicity (pKb) | -4.38 |
| Magnetic susceptibility (χ) | -51.72·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.609 |
| Viscosity | Viscous liquid |
| Dipole moment | 1.7155 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 217.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | –7.7 kJ·mol⁻¹ |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | Precautionary statements: P261, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | Health: 2, Flammability: 1, Instability: 0, Special: -- |
| Flash point | > 106°C |
| NIOSH | WQ6475000 |
| REL (Recommended) | 0.1 ppm |
| IDLH (Immediate danger) | NIOSH has not established an IDLH value for 2-Bromo-5-Formylthiophene. |
| Related compounds | |
| Related compounds |
2-Bromothiophene 5-Formylthiophene 2-Bromo-5-methylthiophene 2-Bromo-5-nitrothiophene 2-Bromo-4-formylthiophene 2-Bromo-3-formylthiophene 2-Chloro-5-formylthiophene 2-Iodo-5-formylthiophene 2-Bromo-5-cyanothiophene |