2-Benzoylthiophene has roots entangled with the maturing field of synthetic organic chemistry. Researchers started focusing on thiophene derivatives in the first half of the twentieth century, hoping to use the unique electronic properties locked within that five-membered ring. Benzoylation of heterocycles became a target for early medicinal chemists since such modifications promised improved biological activities and led to more robust building blocks for pharmaceuticals. As techniques like Friedel–Crafts acylation matured, so did the methods for crafting molecules like 2-Benzoylthiophene, giving scientists a direct way to modify the thiophene skeleton and unlocking broader applications in dye chemistry, agrochemicals, and potential medical agents.
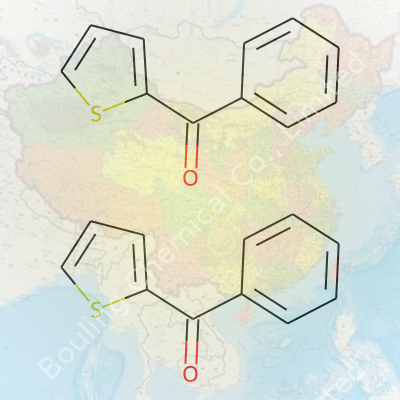
2-Benzoylthiophene stands out as an aromatic compound carrying the formula C13H10OS. Its main feature—attaching a benzoyl group at the 2-position on thiophene—alters not just its reactivity, but the range of end uses. For labs, the compound offers a gateway for new chemical discoveries. The presence of both aromatic rings and a ketone backbone makes it more than an academic curiosity. Batch after batch, I’ve seen researchers reach for it in diverse synthesis projects, from forming more complex molecules to early-stage pharmaceutical work. It even finds some use in the development of organic semiconductors, although those applications are still emerging in academic journals and patents more than on the factory floor.
Most encounters with 2-Benzoylthiophene are visual—from the start it shows up as a pale yellow powder that seems to catch the light differently than simple thiophene or benzophenone. It melts above 50°C, with most literature placing the melting point between 54–57°C. The compound dissolves in organic solvents—ether, chloroform, and even acetone—demonstrating its readiness for lab manipulation. It carries a sweet, almost floral odor if left open in a warm lab, something students notice quickly. Its structure, with conjugated rings, invites questions about electronic delocalization. That explains why 2-Benzoylthiophene absorbs strongly in the UV range and why researchers use UV-Vis spectroscopy to track its presence and purity.
Manufacturers provide 2-Benzoylthiophene labeled with CAS number 2358-05-8. Purity specifics matter: analytical grades often exceed 98% purity, and reputable suppliers provide both HPLC and NMR profiles on request. The safety data sheets (SDS) stress responsible handling, flagging hazards related to eye and skin contact, and inhalation risks during weighing or transfer. The labeling flags its use in labs and not for household applications. Each shipment guarantees batch consistency thanks to strict screening by IR and GC-MS analysis, which in my experience offers peace of mind when embarking on multi-step synthesis. Glass bottles—ambered to cut down on light exposure—help guard against the gradual photodecomposition that can pester those leaving bottles too close to lab windows.
The main path to 2-Benzoylthiophene comes through Friedel–Crafts acylation, using thiophene and benzoyl chloride. Adding a Lewis acid catalyst like aluminum chloride in dry dichloromethane prompts a quick acylation at the 2-position—a lesson familiar to anyone who’s dealt with thiophenes’ distinct electron distribution. Other approaches use milder catalysts or ionic liquids, aiming for greener chemistry and milder reaction conditions, but the classic Friedel–Crafts method still wins for reliability and scalability. After quenching and careful extraction, recrystallization from ethanol often produces analytically pure product, though scaling up brings its own headaches—emulsion-prone washes and the frustrating stubbornness of reaction byproducts sticking around.
On the benchtop, 2-Benzoylthiophene responds well to transformations. The carbonyl group acts as a magnet for nucleophilic additions, such as reductions with sodium borohydride or Grignard reactions to forge alcohols. The molecule tolerates halogenation at the 5-position on the ring or bromination on the benzene segment, which lets chemists build up more intricate molecular backbones. I’ve seen groups tack on alkyl chains at the sulfur for material science work or convert the ketone to an oxime in drug discovery projects. Nearly every research cluster dedicated to heterocycles will have a story or two about tweaking derivatives to try and bump up biological activity, chasing a hint of antibacterial or anticancer promise.
Those searching across databases run into several names: 2-Benzoylthiophene, 2-phenylcarbonylthiophene, or just the abbreviation 2-BT. Some suppliers list it as α-Benzoylthiophene, which points to the same structure—just phrased through different naming conventions. Keeping track helps avoid duplicate ordering or missing key references in patents or journal articles. As science goes global, awareness of alternate translations—like in Chinese or Japanese chemical catalogs—can save days on a literature search or a procurement effort.
Working with 2-Benzoylthiophene calls for respect, not fear. Skin contact causes irritation, and accidental inhalation can start a cough or worse in tightly closed labs. Standard PPE—nitrile gloves, goggles, lab coats—cuts most risk, but good fume hood discipline matters more than many admit. I’ve watched enough beginner errors—accidental spills or clouds of dust when weighing powder—not to underestimate the small hazards of routine lab work. Waste disposal needs the same attention: local standards often make the chemist neutralize or absorb residues before funneling them into regulated chemical waste streams, reducing the chance that downstream processing or water gets contaminated.
Researchers come to 2-Benzoylthiophene because its chemistry underpins wider ambitions: lead molecules for drug screening, new liquid crystal displays, and advanced organic photovoltaics among them. Medicinal chemists value the thiophene core for its presence in antifungal or anti-inflammatory compounds, and the benzoyl group fine-tunes how the molecule interacts with biological targets. Material science researchers leverage its photophysical properties, designing new electronic or light-harvesting layers. In academia, it plays an educational role—students run standard reactions and test purification skills, gaining confidence by handling a 'real' intermediate rather than textbook examples.
Pharmaceutical work tends to orbit around structure-activity relationships: how shifting a group up or down on the ring bends biological outcomes. Recent studies dive into growing selective inhibitors for kinase enzymes or seek to tame neurodegenerative processes by modifying the benzoyl or thiophene ring. Material research harnesses the molecule’s stability under controlled conditions to build better field-effect transistors or sensors sensitive to gases or pH. Academic clusters in Japan, China, and Europe push greener synthesis methods, using alternative solvents and catalysts. The pressure to build more sustainable chemical processes flows directly from student benches to published patents and, sometimes, to new commercial products.
There’s still plenty to learn about toxicity. Animal model testing points to moderate acute toxicity, mostly through inhalation and skin exposure at higher doses. Chronic exposure studies linger in the background of regulatory filings, with most data suggesting caution but not immediate hazard in standard laboratory use. Environmental persistence hasn't generated major concern due to ready breakdown in the environment, yet as new derivatives get made and as industrial application scales up, long-term safety tracking becomes essential. I’ve seen grant applications get stuck until a full suite of toxicology data landed, which shows the tightrope researchers walk between progress and safety oversight.
Interest in 2-Benzoylthiophene only grows as cross-disciplinary teams seek molecules that do double or triple duty. Collaboration between synthetic chemists, biologists, and engineers promises new composites, better pharmaceuticals, or even next-gen OLED devices. Improvements in synthetic methods could reduce hazardous byproducts and slash energy consumption, meeting tighter green chemistry rules and making the compound more sustainable for widespread use. As material requirements evolve for electronics and as biological testing gets more rapid and nuanced, the flexibility of this molecule hints at roles not even guessed at a decade ago. Progress here means working at the sharp edge where historical knowledge, day-to-day lab reliability, and tomorrow’s applications all push together, each hoping to get the best out of a simple but versatile aromatic compound.
Ask most people on the street, and 2-Benzoylthiophene will sound like something from a sci-fi movie. The name doesn’t roll off the tongue, but in chemistry labs, its story reaches a lot further. In my early college days, the first time I came across it, I was sweating through an organic chemistry exam. Over the years, though, I saw this compound pop up beyond textbooks—sometimes even in places you wouldn’t expect.
Most folks working with this molecule use it as a building block. It's a workhorse in organic synthesis, especially where they want to craft new drugs or more effective dyes. Some of the world’s popular painkillers and anti-inflammatory medications have roots in compounds that are close chemical cousins to 2-Benzoylthiophene. Researchers need it to build complex molecules step by step, almost like assembling Lego sets. Each time I handled it during my research internship, I could see why: it reacts predictably, and it opens the door to other chemical changes that save researchers days, even weeks.
Drug companies look at 2-Benzoylthiophene not just as a tool, but as a gateway. It often helps spark the creation of new medicines. For example, the core structure of 2-Benzoylthiophene shows up in molecules studied for their cancer-fighting or anti-infective abilities. That benzoyl group sticking to the thiophene ring isn’t just for show—it changes how the rest of the molecule behaves. In labs, even small tweaks to that structure can lead to big changes in how a new drug works or gets absorbed in the body.
It doesn’t stop with medicine. Some specialty dyes use thiophene-based chemicals to achieve strong colors resistant to fading. I've seen research proposals harnessing these compounds for solar cells, chasing more efficient ways to turn sunlight into usable energy. The backbone provided by 2-Benzoylthiophene gives chemists flexibility to experiment without starting from absolute scratch.
Of course, handling any chemical comes with responsibilities. Even though 2-Benzoylthiophene isn’t as hazardous as some more notorious compounds, it still pushes for care. Touching it with bare hands can irritate skin; breathing in the fumes is no joke. It needs smart storage and disposal—nobody wants a spill, especially near groundwater. Back at my old lab, proper gloves, fume hoods, and careful tracking made the difference between safe research and a dangerous mess.
Scale matters, too. While academic labs handle small volumes, industry work can involve barrels. Pushing for greener chemistry, researchers keep searching for ways to reuse 2-Benzoylthiophene fragments after finished reactions. Newer processes aim to waste less and reduce the use of harsh solvents. Supporting grants and policies that reward less toxic chemistry—and giving students hands-on safety training—pulls the industry forward.
2-Benzoylthiophene might never make the front page, but its fingerprints are everywhere, from early-stage drug discovery to making better, longer-lasting technologies. The world keeps spinning, and so does chemistry—sometimes thanks to compounds like this that quietly keep progress moving forward.
Chemistry often boils simple questions down to numbers and letters, but living with these compounds daily—be it in the lab or reading chemical labels—gives them a familiar face. 2-Benzoylthiophene comes with the molecular formula C11H8OS. This isn’t just a string of elements; this composition hints at its structure and clues about its behavior.
Its molecular weight stands at 188.25 g/mol. You pick up a bottle, and the number matters: dosing, reactions, and calculations depend on those grams. Anyone who’s ever miscalculated molar mass even once—finding that a planned reaction goes off-kilter—learns quickly why each subscript in a formula counts. In labs, that number is as essential as the safety glasses on your nose.
2-Benzoylthiophene turns up when you dive into research around organic synthesis and material science. Its appeal comes from the way the benzoyl group hooks up with the thiophene ring. That combination brings both structure and reactivity, making it useful as a building block.
The thiophene ring alone gets a lot of attention as a common motif in drugs, dyes, and organic electronics. With the benzoyl group attached, reactivity shifts. Chemists reach for it not just for what it offers on its own, but for how it can be shaped into something bigger—a precursor to more complex molecules with targeted properties.
Sliding into the practical side, molecular weight isn’t just trivia. Ever prepared a solution and watched a reaction flop due to a wrong calculation? I once mixed up compounds for a polymerization, mistaking molar mass by just a few points, and the polymer wouldn’t form at all. That mistake cost hours. Each decimal matters. Sometimes a misplaced hydrogen or forgotten oxygen changes the outcome entirely.
For those handling precise reactions or developing drugs, that 188.25 g/mol turns into a decisive figure. Every synthesis starts at the scale, and accuracy matters from the very beginning. I’ve seen teams recalibrate balances just to ensure that this small piece of math won’t unravel an entire day’s work.
Errors in formula or weight go beyond a grade in school. In research settings, these details can damage credibility or waste rare, expensive reagents. Precision wins the day in chemistry, and tools make a difference. Modern digital scales have made life easier, but mistakes still happen.
Standardizing training helps, but nothing replaces a careful double-check. Peer review in the lab, much like in publishing, often catches these slip-ups. Cross-checking with colleagues or using reliable reference databases can keep things on track. Software for chemical calculations has helped many fumble less with numbers.
Chemicals like 2-Benzoylthiophene may never make household news, but understanding their properties, even down to molecular weight, keeps experiments running reliably—and science moving forward. Trading stories in the lab about those times formulas didn’t add up reminds me: behind every successful experiment, there’s always attention to the basics.
2-Benzoylthiophene doesn’t draw much attention outside of labs or specialized industries, but its handling deserves care. This compound isn’t just another reagent to toss in a corner. Over time, I’ve seen mistakes with storage lead to ruined batches, unneeded expenses, and even safety scares that should’ve been avoided.
Let’s be honest—most people working day-to-day with chemicals want fewer headaches, not more. It’s tempting to judge something harmless by appearance. But 2-Benzoylthiophene isn’t water or baking soda. Its structure brings both value in synthesis processes and hazard if ignored.
Early in my chemistry days, a colleague left a bottle of a similar compound by a sunny window. No explosion, nothing dramatic—just a subtle change in color, followed by a frustrating call to reorder material that should’ve lasted for months. The lesson sticks: chemicals react quietly to temperature, light, and air.
With 2-Benzoylthiophene, moisture and heat start to chip away at quality. Impurities sneak in, and pretty soon that crystal-clear solid isn’t so pure. A careless approach doesn’t just eat profits; it risks lab results and can nudge health concerns higher. Stashing it in a warm or damp storage closet isn’t about convenience—it’s a shortcut to spoiling your investment.
Experience tells me that dried, sealed, and dark containers aren’t negotiable luxuries—they’re the cost of doing business right. The best labs I’ve worked in keep 2-Benzoylthiophene somewhere cool, usually between two and eight degrees Celsius. Not freezing, not sweating. Just steady. The container stays closed except during brief, intentional sampling or weighing.
Amber glass bottles work best. Light doesn’t reach the compound so easily, and glass won’t react like some plastics might. If you catch whiffs of unfamiliar smells, or see color shifts you can’t explain, assume contamination. Don’t take chances, even on slow days or tight budgets. Better safe than a chain of ruined reactions.
Some folks think labeling and dated logs look bureaucratic—until they can’t recall when a reagent landed on their shelf. I’ve watched teams scramble through handwritten notes, only to toss out a half-full container because the source and date became a mystery. Shortcuts in record-keeping mean wasted resources and nagging doubt about results.
It pays off to log everything: source, arrival date, and any observed changes over time. Pair this with organized shelving, free from caustics, acids, or open containers that invite cross-contamination, and workplace drama drops sharply. The environment stays predictable, projects finish on time, and everyone breathes easier.
Storage isn’t one-and-done. Regular checks prevent nasty surprises. I recommend monthly walk-throughs; a fresh set of eyes will spot leaks, condensation, or accidental mixing before small problems get bigger. In fast-paced or multi-user spaces, weekly check-ins catch even minor slip-ups, saving frustration later.
No fancy equipment required: a flashlight, a reliable logbook, and a clear policy that makes it everyone’s job are enough. If someone’s new to the chemical, hands-on training beats any written memo. Seeing the setup, practicing safe handling, and understanding why each rule exists lays a foundation that sticks.
No one likes preventable setbacks. Respecting chemicals like 2-Benzoylthiophene means more than following rules—it means thinking ahead, spending a few extra moments on labels, lids, and logs, and treating every storage step as insurance. That care ripples out, saving money and sweat long after the bottle hits the shelf. That, to me, spells progress.
Working with chemicals every day, I’ve come to respect the quirks of each compound. 2-Benzoylthiophene doesn’t look threatening at first glance—white crystals, faint odor, slips into solvents easily. Don’t let that calm face fool you. A quick look at its data sheets shows it’s no sugar. It’ll sting your eyes, make your skin burn, and a deep breath of dust can turn your lungs scratchy. The molecular formula, C9H6OS, hides sulfur and a benzoyl group, both of which I’ve learned can dial up reactivity and add risk when you least expect it.
Going by the stories from classmates and a few close calls in shared spaces, this compound deserves full respect. The Material Safety Data Sheet (MSDS) marks it as harmful if inhaled, swallowed, or after skin contact. Eye contact triggers redness and pain; skin exposure can lead to dermatitis. Powdered organic compounds like this often behave unpredictably near open flames or static because their particles float. Every chemist I know has seen a surprise puff, strong enough to panic a room, with people rushing for running water.
You really have to get your habits right. Even though 2-Benzoylthiophene isn’t classed as a major health hazard or deadly poison, the risk stacks up if care gets lazy. Open lab coats, bare hands, uncovered faces—these guarantee trouble. One guy in my department ended a night in the emergency room after brushing chemical dust from his jeans without gloves. After that, gloves went on no matter how quick the task, goggles snapped in place, and fume hoods never stood idle. The compound can get into lungs or eyes, and some say long-term exposure could even have chronic effects that science hasn’t fully nailed down yet.
Add to the mix its flammability. 2-Benzoylthiophene lights up at high heat, and if you’ve ever seen smoky, acrid air after a flask mishap, you know why even the ordinary-seeming powders need remote storage. Airtight jars, kept cool and dry, far away from oxidizers, stop this chemical from causing accidents. Every so often, a new intern skips the dry gloves or tries pouring too quickly—spills get everywhere, static on a plastic spatula throws sparks, and you have a fire drill no one wants to repeat.
Precaution beats cure every time. No great mystery—lab coats, fitted nitrile gloves, chemical goggles, and working inside a ventilated hood cover most of the risk. You keep your workstation clean, store small portions in labeled vials, wipe down surfaces, and you stay ahead of trouble before it knocks. Proper waste disposal keeps the environment safe, too. It only takes a forgotten fragment on a gloved finger to start trouble, so awareness and strict routine turn new risks into familiar, controlled routines.
Better training for staff and students solves most problems long before they occur. Some workplaces now use color-coded containers and air-quality monitors where dry compounds get weighed, cutting down on accidental exposure and inhalation. Well-marked spill kits and regular drills make sure everyone feels ready if something tips or leaks. Regulators still push for tighter guidelines, especially for chemicals with poorly studied effects, but much of safety grows from old-fashioned discipline and mutual reminders. Comfort in handling grows with regular respect, not from shortcuts or disregard, and no step feels wasted when lab members head home with all their senses and skin unharmed.
Pharmaceutical laboratories don’t often get the spotlight unless they’re announcing miracle drugs or headline-grabbing breakthroughs. But behind the scenes, chemists rely on certain building blocks that make entire drug classes possible. 2-Benzoylthiophene steps up as one of those workhorse ingredients, showing up in syntheses for many well-known medications. It gets used in the process for making anti-inflammatory drugs—like some versions of ibuprofen—and contributes to substances used in treating conditions from bacterial infections to epileptic seizures.
Some might imagine this chemical as sitting around in test tubes, but, in reality, it’s at the heart of reactions and transformations. Chemists like my friends working in generic drug manufacturing say that reliable access to reagents like 2-Benzoylthiophene can save months in R&D. Without it, some synthesis routes just stall. Rollouts of new medicines, generics, or even just incremental improvements depend on these ‘background’ chemicals.
Walk into any department store and take a deep whiff in the perfume aisle; you’re probably picking up traces of chemistry like 2-Benzoylthiophene. Its aromatic structure means it serves as a core compound for creating new scents. It doesn’t smell great on its own, but it helps bind, stabilize, and make other fragrance ingredients pop. I had a summer job packing gift sets in a perfume factory, so I saw fragrance teams routinely relying on niche chemicals like this to tweak new blends until they hit just the right note.
The food world isn’t left out, either. Synthetic flavors, especially for baked goods or chocolate, sometimes include derivative compounds. Careful control in manufacturing ensures these additives stay at safe, well-studied levels. Headlines warn us about artificial this-or-that, but many of those news stories skip the care and precision chemists put into food safety and testing.
Materials science might sound far removed from daily life, but applications get closer to home than most think. 2-Benzoylthiophene shows up in the patented recipes for making specialty polymers. These polymers give certain plastics, coatings, and electronic components their unique features. My cousin, who works at a plant producing flexible circuit boards, mentioned how tweaks in the chemical formula—sometimes using just a hint of 2-Benzoylthiophene—affect resistance, color, and flexibility.
For people working on solar panels or organic electronic devices, innovations often depend on molecules like this. Greater efficiency in solar cells and displays starts with the foundational level, where these specialty compounds help craft improved conductive layers. It reflects the broader world of chemistry innovation—progress made in small, crucial steps, not just flashy inventions.
Demand for 2-Benzoylthiophene isn’t about to disappear, but keeping its production safe and environmentally sound presents issues. Old-school syntheses tended to give off plenty of waste, some of it hazardous. Talking to a friend employed in green chemistry, there’s a clear push toward more sustainable methods. Companies now invest in cleaner, more efficient production routes, minimizing toxic byproducts.
Supply issues crop up, too. As global supply chains stumble, cost and access concerns for key chemicals trickle down to everything from pills to circuit boards. Some teams try to develop alternatives that fill the same roles, but switching isn’t always straightforward. Demand for specialty chemicals keeps pressure on chemical suppliers to deliver both quality and consistency, without the usual excuses for delays or subpar batches.
If nothing else, looking at 2-Benzoylthiophene’s journey serves as a reminder: the tiny things in the background can make a huge difference to things we use every day. From keeping medicine cabinets stocked to helping new tech become reality, these molecules deserve a bit more credit—along with careful regulation and smarter production, not just for science’s sake but for the long-term health of everyone who depends on what they help create.
| Names | |
| Preferred IUPAC name | (1-thiophen-2-yl ethan-1-one) |
| Pronunciation | /tuː bɛnˈzɔɪl ˈθaɪ.oʊˌfiːn/ |
| Identifiers | |
| CAS Number | 2131-73-5 |
| Beilstein Reference | 1208735 |
| ChEBI | CHEBI:211691 |
| ChEMBL | CHEMBL22746 |
| ChemSpider | 81698 |
| DrugBank | DB08395 |
| ECHA InfoCard | 13b4ea49-3a12-4364-8bc6-db8753489624 |
| EC Number | 201-583-4 |
| Gmelin Reference | 821517 |
| KEGG | C09866 |
| MeSH | D015869 |
| PubChem CID | 6971 |
| RTECS number | KM5775000 |
| UNII | 2M11HF29HA |
| UN number | UN3439 |
| Properties | |
| Chemical formula | C11H8OS |
| Molar mass | 206.27 g/mol |
| Appearance | White to yellow crystalline powder |
| Odor | Odorless |
| Density | 1.204 g/cm³ |
| Solubility in water | insoluble |
| log P | 2.9 |
| Vapor pressure | 0.00163 hPa (25 °C) |
| Acidity (pKa) | 12.71 |
| Basicity (pKb) | 13.01 |
| Magnetic susceptibility (χ) | -81.0e-6 cm³/mol |
| Refractive index (nD) | 1.6360 |
| Dipole moment | 3.47 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 352.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | 90.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -219 kJ/mol |
| Pharmacology | |
| ATC code | N02CX06 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302 + H312 + H332: Harmful if swallowed, in contact with skin or if inhaled. |
| Precautionary statements | Precautionary statements: P261, P264, P271, P272, P273, P280, P302+P352, P305+P351+P338, P312, P321, P332+P313, P333+P313, P337+P313, P362+P364, P501 |
| NFPA 704 (fire diamond) | 1*2*0 |
| Flash point | 69 °C |
| Autoignition temperature | 285 °C |
| Lethal dose or concentration | LD50 (oral, rat): 590 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 > 5000 mg/kg |
| NIOSH | BZJ122 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 100 mg |
| Related compounds | |
| Related compounds |
2-Acetylthiophene 2-Benzylthiophene 2-Phenylthiophene Benzothiophene 3-Benzoylthiophene |