Years ago, chemists spotted the potential in pyrrole rings, those five-membered, nitrogen-containing rings tucked into the world of heterocycles. By the mid-20th century, researchers began adding acyl substituents, such as benzoyl, to tweak physical and chemical traits. Back in labs powered by hot plates and glassware, early syntheses of 2-Benzoylpyrrole set off a wave of curiosity. Journals chronicled modest yields, and pioneers reported how challenging it felt to gain pure, well-characterized samples. Fast forward, those early efforts paved the way for streamlined protocols, finer analytical techniques, and solidified 2-Benzoylpyrrole as a common intermediate in both academic and industrial environments.
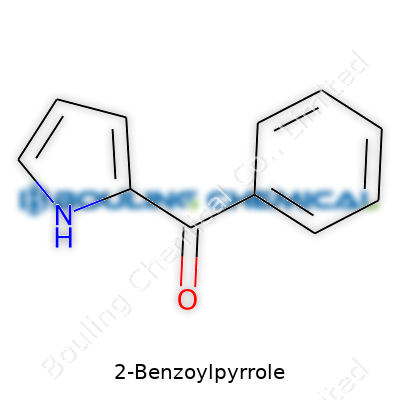
2-Benzoylpyrrole stands as an organic compound where a benzoyl group attaches squarely at the second position of the pyrrole ring. In practice, it looks like a pale yellow powder or almost colorless crystalline solid, easy to spot in a well-lit flask. Chemists value it for stability on the shelf and reactivity on the bench. Its structure carves out a distinct niche — reactive enough for derivatization but not so fragile that simple storage turns into a headache. Across catalogues and in chemical storerooms, the compound pops up thanks to versatility in both fundamental research and as a gateway to more complex molecules.
At room temperature, the solid sits with a melting point that hovers around 120°C, though slow heating reveals some batches might shift a few degrees higher. As a mid-sized, aromatic compound, it dissolves well in organic solvents such as chloroform, dichloromethane, and ethanol, yet resists blending thoroughly with water. Its moderate volatility turns noticeable only on strong heating. Under first glance, the scent is faint, somewhat reminiscent of other simple pyrroles — not pungent, but unmistakable if you know what you’re looking for.
Chemically, 2-Benzoylpyrrole features electrophilic and nucleophilic sites, with the carbonyl group eager for reaction and the aromatic ring providing delocalized electrons. In UV-vis spectrometry, the benzoyl group shifts absorption peaks, a neat trick that aids identification during routine purity checks. Those properties make it reliable not just in standard reactions but as a benchmark in method development.
On arrival from a supplier, the label details CAS number 38561-74-9, molecular formula C11H9NO, and molar mass close to 171.20 g/mol. Safety sheets warn of mild irritation upon direct contact and standard cautions about avoiding unnecessary inhalation or ingestion. Many bottles show a purity of 97% or higher, often certified by NMR and GC-MS spectra included with batch paperwork. Color, melting point, and solubility range all get listed to help chemists avoid confusion with similar analogs. Glass bottles with airtight seals remain the norm, avoiding light and moisture.
Synthesizing 2-Benzoylpyrrole generally relies on acylation of pyrrole, with benzoyl chloride serving as the acyl source. The Friedel-Crafts acylation route leads the way, using Lewis acids like aluminum chloride as catalysts. Students in teaching labs quickly learn the exothermic kick these reactions bring, urging slow addition and generous cooling. After reacting, extra steps remove aluminum salts and purify the organic product — washing, organic extraction, and rotary evaporation follow a familiar script. Some modern protocols substitute milder conditions or greener solvents, yet the core steps rarely change much, drawing from decades of fine-tuning.
With its reactive carbonyl and aromatic core, 2-Benzoylpyrrole transforms in skilled hands. Reduction of the carbonyl produces 2-phenylpyrrole, expanding the compound’s value as a building block. Electrophilic substitution at the remaining pyrrole positions gives a long list of derivatives, each with distinct potential in either pharmaceuticals or advanced materials. Even basic modifications such as N-alkylation open new routes. Certain research groups lean into cross-coupling or cyclization reactions, showing off the compound’s versatility. Whether constructing more elaborate heterocycles or introducing functional handles, 2-Benzoylpyrrole rarely stays unaltered for long in a creative environment.
Databases sometimes call it 2-benzoyl-1H-pyrrole or 1H-pyrrole-2-yl phenyl ketone, and catalogues adopt these labels to cover global naming standards. Commercial products reflect both naming systems, so slight confusion can set in if you jump from one supplier to another. It underscores the importance of double-checking molecular structures on datasheets, especially during procurement for multi-lab projects.
Although many treat it as an ordinary lab chemical, careful handling matters. Skin contact or accidental inhalation brings mild irritation for most people. Wearing gloves and working in a fume hood sidestep these risks. Store the compound in dark, dry spaces to hold off degradation. Laboratories treating it as a controlled precursor stick to robust inventory and waste tracking. On the shop floor or in manufacturing, proper PPE and spill control get emphasized to match health and environmental guidelines. Following established chemical hygiene plans, users can use 2-Benzoylpyrrole safely with no trouble.
Pharmaceutical researchers rely on 2-Benzoylpyrrole as a core intermediate in the synthesis of anti-inflammatory and antiviral agents. Its structure encourages incorporation into small-molecule libraries during drug discovery, where adjustments at the benzoyl or pyrrole ring quickly produce new analogs. Material scientists draw on it for assembling advanced organic semiconductors or light-harvesting systems, as the aromatic framework underpins electronic communication across molecules. Some specialty dye producers even look to it when aiming for stable, lightfast pigments. By standing at the intersection of chemistry innovation, 2-Benzoylpyrrole finds itself useful whether chasing better medicines, sensors, or new electronic materials.
Research groups continue to explore downstream modifications, using new catalysts or smarter solvents to claim higher yields and improved selectivity. Many set their sights on asymmetric synthesis, aiming to control product chirality with ever-finer precision. Academic poles focus on reaction mechanisms and computational models, mapping how structure influences function. On the industry side, teams look for scalable syntheses with minimal byproducts, harnessing process intensification schemes once limited to bulk commodities. The compound’s balance between reactivity and stability keeps it relevant for researchers intent on discovering new reactions or scaling up promising pharmaceuticals.
Most available data point to low acute toxicity for 2-Benzoylpyrrole, though comprehensive chronic studies remain sparse. Standard cell culture assays detect limited cytotoxicity compared to related chemotypes, though caution kicks in since metabolic products of pyrrole derivatives can occasionally produce reactive intermediates in vivo. Animal studies at high doses note mild hepatic alteration without progressive damage, yet repeated long-term exposure remains less understood. Regulatory flags center on fine particulate handling and broader environmental disposal, especially since downstream reactions could yield new hazards. To fill knowledge gaps, more in-depth toxicology rounds are warranted, especially as the compound’s uses branch out into consumer products.
Interest in 2-Benzoylpyrrole continues to climb as the challenges of drug resistance and demand for lighter, more efficient materials mount. Properly tuned, its core structure unlocks next-generation pharmaceuticals—especially where heteroaromatics serve as privileged scaffolds. In the technology sector, increased research on organic electronics pulls more eyes toward pyrrole derivatives, given their conductivity and processability. Sustainability questions motivate the search for greener synthetic routes, greener solvents, and reduced waste streams. As scientists push for higher efficiency and smarter design, 2-Benzoylpyrrole remains both an enduring foundation and an open invitation for innovation. Without a doubt, its journey will grow with every research project and industrial breakthrough.
2-Benzoylpyrrole packs quite a punch for such a small compound. Think about it: on the outside, it looks like so many other chemicals, just another name in a massive textbook. Dig deeper, and a clear personality starts to emerge, shaped by its very structure. Walk through any chemistry class or research lab, and someone will sketch a benzene ring, a pyrrole, or maybe both. 2-Benzoylpyrrole puts these together—one benzene ring forms a strong partnership with pyrrole via a carbonyl bridge right at the number two spot on the pyrrole ring.
Some call chemical structure the soul of a molecule, and here it shows. The benzoyl group isn’t just eye candy—it connects straight into the business end of pyrrole. To picture it, start with pyrrole, a five-membered ring with a nitrogen atom at the top. Count around the ring; stick the benzoyl group (a benzene attached through a carbonyl) onto the second carbon. It is a structure that whispers versatility in synthesis and reactivity. Chefs talk about basic ingredients; chemists treat these building blocks in much the same way.
In the lab, I remember mixing pyrrole derivatives in glassware that smelled vaguely of almonds, only for the supervisor to swing by, peering over glasses, and ask about the placement of functional groups. Add a benzoyl in the wrong spot, and suddenly the expected transformation goes nowhere. Hit the sweet spot at position two, and chemistry starts to sing. Synthesis using the Friedel-Crafts acylation jumps to mind. Here, an acyl chloride and a little help from a Lewis acid bring pyrrole and benzoyl together in a strong, direct bond. If you’ve ever spent too long waiting for a reaction to run, you appreciate how structure like this leads you down the right paths.
Small molecules with this sort of scaffold often end up in pharmaceutical research. Compounds resembling 2-Benzoylpyrrole sometimes show up as intermediates in the synthesis of biologically active agents. Think anti-inflammatory candidates, precursors for dyes, and more. When a compound sports both a benzene and a nitrogen-containing ring, it nudges its way into the attention of medicinal chemists. The benzoyl group adds rigidity, which can mean tighter binding to active sites in enzymes. Pyrrole rings breathe some flexibility into the mix, letting the molecule fit into all sorts of tiny protein pockets.
Sourcing pyrrole derivatives rarely proves easy. Some batches arrive unreliable, sometimes with mystery impurities. Purification often takes days and lots of checking. It's tempting to cut corners, but walking that fine line rarely ends well. Companies and academics alike could invest more in cleaner routes—greener solvents, direct routes, and catalysts that avoid waste. The payoff? Not just better chemistry, but less headache for everyone involved.
2-Benzoylpyrrole reminds us how a simple tweak, a change at just one spot, can shift an entire molecule’s fate. Too often, focus lands on big flashy structures, but the real stories often start small, right in the details of chemical structure.
Working with organic compounds often feels like living in two worlds—one where you tinker with tiny molecules, another where industry looks to apply these discoveries in ways you can actually see. 2-Benzoylpyrrole isn’t a household word, but its fingerprints show up in some surprising places. My own time in a cramped, overworked lab taught me that this compound can turn heads in more than one field.
Anyone who’s handled drug research knows the pressure to innovate. You’re constantly on the lookout for new building blocks, hoping one unlocks a better treatment. 2-Benzoylpyrrole shows up here as a strong candidate for cranking out new biologically active compounds. Medicinal chemists grab it for its adaptable ring structure, using it as a scaffolding for designing everything from anti-inflammatory agents to cancer drugs. I remember late nights at the bench, mapping out fresh derivatives based on this skeleton, weighing activity against toxicity, and jotting notes in the margin—sometimes those scribbles led right to a promising lead compound.
Think of all the materials around us that owe their properties to careful molecular tweaks. 2-Benzoylpyrrole can serve as a monomer or a component in complex polymers and specialty dyes. In grad school, I watched colleagues mix up batches of materials with unusual mechanical strength or vivid color—some of which started with this molecule. Specialty plastics, OLED materials for display tech, and even specific photo-reactive coatings aren’t as fancy without such ingredients. This opens a door for materials scientists chasing products that don’t just meet, but set, new standards.
Modern farming leans heavily on chemistry, and that’s where compounds like 2-Benzoylpyrrole pay their rent. Its basic structure makes it a favorite for tweaking into molecules that fight off pests and weeds. I saw projects where chemists would start with 2-Benzoylpyrrole to build a new line of insecticides, hoping for fewer side effects on crops and the ecosystem. The stakes are big—farmers lose less harvest to pests, and local water doesn’t get as tainted by run-off.
There’s always a flip side. Sourcing 2-Benzoylpyrrole or making it cleaner isn’t easy. Handling waste by-products used to mean just dumping them. Cleaner syntheses and greener chemistry approaches help address this. Switching to catalysts that reduce toxic leftovers, seeking solvents that break down safely, and recycling steps in the production process can reduce a lot of headaches. From the ground up, chemists working with 2-Benzoylpyrrole have pushed for solutions that look out for lab safety and the river downstream.
People working with 2-Benzoylpyrrole want it to be a springboard for discovery, not just a lab curiosity. Chemists keep finding ways for its structure to trigger new applications, and industries pick it up to design better products and treatments. With each new challenge—safer drug design, longer-lasting materials, smarter farming—every mind in the room adds another chapter to its story. That’s the grind, and sometimes the magic, of chemistry.
People poking around in organic chemistry often bump into 2-Benzoylpyrrole—a compound with a look and a level of purity that says a lot about its origins and intended use. For anyone who’s worked a little with aromatic compounds, the basics of color, texture, and cleanness carry big weight. Talking about 2-Benzoylpyrrole, it usually comes as a pale yellow solid, sometimes more toward off-white, depending on the route of synthesis and the choices made along the way. Once, when preparing a batch during graduate research, the yield would have given me textbook-grade if not for a faint blush of yellow. Turns out, even small tweaks in purification can shift the appearance.
Physical Appearance Isn't Just Cosmetic
It’s not just about looks—color gives clues. If the powder shows too much yellow or veers brown, that often points to impurities, leftover reagents, or byproducts from side reactions. Having handled plenty of similar compounds, I could tell you right away: a pure 2-Benzoylpyrrole sample almost glows with a muted translucence. Run your finger through it, and you can feel it's not waxy or oily, but more powdery, almost crystalline at times. If the texture turns sticky or clumpy, moisture or contaminants have crept in.
Checking Purity in the Real World
Purity matters because anything less than about 98% can throw off research results or downstream syntheses. Discoloration isn't just annoying—it might derail an experiment if reactive species linger. Labs check purity by running melting point analyses, thin-layer chromatography, and the classic NMR spectroscopy. Melting points in the 133 to 135°C range usually spell a tidy product. The smoother that number lines up with the literature, the cleaner your batch.
In one industrial setting, a colleague and I saw a batch turn a suspicious yellow-brown. We dug in and found the culprit lurking as a carryover solvent from a rushed distillation step. Unappealing, sure, but more importantly, it distorted downstream tests and added an almost burnt smell—clear red flags. Even for a commodity compound, that kind of slip didn’t fly. We doubled up on purification, taking the time for slow recrystallization, and the compound returned to its pale, trusted form.
Impurities mean more than a dip in laboratory pride. In pharmaceutical research or specialty materials, even a trace of the wrong stuff leads to unreliable results or risk down the line. Say someone in drug discovery swaps 2-Benzoylpyrrole from two sources—a 95% and a 99% pure sample. They quickly see one gives wobbly data, the other smooth curves in their chromatograms. The difference? Those hard-to-see contaminants act like sand in the gears.
Solving the problem often comes down to patience: recrystallization, using activated charcoal to soak up colored junk, or running extra chromatography columns to weed out the holdouts. The work may slow down, but junky samples make data worthless. A mentor once told me, “Rushing purity means doubling your headaches.” He was right. Investing in careful, thorough cleaning pays off in cleaner data and less hands-on troubleshooting later.
Folks working with 2-Benzoylpyrrole—or any sensitive intermediate—learn fast that purity and appearance are not low-priority afterthoughts. They signal the path ahead, hint if you should keep trusting a sample or start over. That mindful attention gives chemists confidence, whether they’re chasing a new reaction or scaling up for something much bigger.
Most folks don’t deal with things like 2-Benzoylpyrrole every day. Still, this powder shows up in research labs, chemical shops, and sometimes as an intermediate in making pharmaceuticals. The moment a new batch rolls off the docks, safety should jump to the front of everyone’s mind. There’s this natural urge to get started with your work, but one spilled container can put a whole project on hold—or send someone to the doctor.
People often glance at the label, toss a jar onto the nearest shelf, and count that as storage. I learned early in my own lab days that some compounds don’t forgive these shortcuts. 2-Benzoylpyrrole thrives in cool, dry places. Moisture—especially in summer—can turn a clean white powder into stiff, clumpy stuff that refuses to dissolve or weigh out neatly. Heat messes it up just as much, sometimes making it break down before you even use it. Tossing a container in a regular cabinet puts your material and your work at risk.
Storing it in a controlled cabinet, out of direct sunlight, actually saves money and hassle in the long run. Everyone grows tired of issuing new purchase orders for ruined chemicals. I’ve also noticed that access should stay limited. Locking up the container keeps people who don’t recognize the label from accidentally getting exposed. Training counts too—labels and dates help, but the real safety comes from every hand that grabs a bottle knowing what they’re about to touch.
It’s all too easy to get lazy with gloves and goggles after working with benign stuff. But every chemical handler has had that moment—the itch on an eyebrow mid-transfer, the sudden tickle on your arm. Nitrile gloves, a fitted lab coat, and goggles become standard for anything not food-grade. I got a splash once from a similar compound because I was too quick to handle it bare-handed. The itch and red skin lasted hours, a lesson that sticks far longer than a printed manual. Gloves only work if you check them for rips first, and a mask or respirator helps in case you generate dust.
More spills get cleaned up wrong than people admit. Scooping up powder with makeshift paper isn’t enough. Using a vacuum with proper filters or specialized absorbent pads keeps the mess contained. Dumping powder in a regular trash can just spreads risk, especially if janitorial staff aren’t warned. Disposal matters as much as safe storage. I’ve watched people skip the neutralization or special waste containers, and it almost always leads to more problems down the line.
Taking storage and handling seriously means fewer lockout days, better yield, and far less drama. The work environment feels less tense when everyone knows you aren’t playing roulette with their health. I once introduced a sign-off sheet for chemical access, just to keep track. Nobody loved it at first, but accidental exposures dropped almost to zero. People eventually trusted the process. That’s what really keeps a lab moving: not the compounds themselves, but how well we care for each other while using them.
It’s easy to overlook the significance of tiny numbers in a chemical name, but for folks who work in a lab—or even people just curious about things—every digit matters. Take 2-Benzoylpyrrole, for example. The molecular weight clocks in at 183.2 g/mol, and its CAS number is 2644-45-9. These aren’t just random figures hanging in space. They unlock specific doors, both in research labs and on factory floors.
If you're ever flipping through a chemical catalog or prepping for a bench experiment, these numbers guide you to the right bottle among thousands. In my grad school days, I learned the hard way how grabbing the wrong compound, thanks to a similar name or structure, can waste a week of work. The molecular weight can save you from that headache. It checks your math and keeps your yields accurate. Something like a miscalculation could knock an entire project sideways, especially for students under pressure.
People don't just stumble on this compound randomly. Chemists explore 2-Benzoylpyrrole to push boundaries in drug discovery and advanced materials. Its structure—pyrrole ring bonded to a benzoyl group—opens doors to new molecules that target disease or deliver performance in organic electronics.
The CAS number may seem like bureaucratic trivia, but it’s more like a universal identification card. Even a slight variation in structure—say, switching the benzoyl group to position 3—wouldn’t just change its name. It’d transform its identity in global databases. I remember hunting down rare chemicals for a synthesis project; a single incorrect digit in the CAS number had me talking to vendors from three different continents. Time spent untangling that mistake could’ve gone into building or testing something new.
Tracking the right molecules isn’t as simple as looking up their names. Chemical suppliers juggle thousands of compounds, and scientists sift through countless articles every year. Typos or small errors in documentation disrupt supply chains, delay research, and sometimes lead to safety risks. Imagine ordering a kilogram of what you think is 2-Benzoylpyrrole, only to unpack the wrong isomer. Lab safety goes out the window.
One low-tech but effective trick: always triple-checking both the CAS number and molecular weight before ordering or using a chemical. For labs dealing with lots of compounds, digital inventory systems with barcode scanners take much of the human error out of the equation. Open databases like PubChem or ChemSpider aren’t perfect, but they’ve made chemical reference smoother than ever. University labs post “cheat sheets” near their ordering computers, reminding everyone to vet CAS numbers before clicking ‘purchase’—experience shows this step saves more than a bit of hassle.
Some might say that the precise molecular weight or a number assigned to a compound doesn’t change daily life. Still, the ripple effects reach further than people think. Every drug, plastic, or paint starts with exact chemistry. A misplaced number can mean months of research disappear, or a major production run goes sideways. That weight—183.2 g/mol—and CAS number—2644-45-9—are small hooks that keep entire industries anchored in accuracy. As someone who’s had to explain a delay because the wrong chemical showed up, I know how much rides on getting these details right from the start.
| Names | |
| Other names |
1H-Pyrrole, 2-benzoyl- 2-Benzoyl-1H-pyrrole 2-Pyrroylbenzene |
| Pronunciation | /tuː bɛnˈzɔɪlˈpɜːr.oʊl/ |
| Identifiers | |
| CAS Number | [4511-42-6] |
| Beilstein Reference | 2065907 |
| ChEBI | CHEBI:73312 |
| ChEMBL | CHEMBL2007613 |
| ChemSpider | 115668 |
| DrugBank | DB07773 |
| ECHA InfoCard | ECHA InfoCard: 100.039.873 |
| EC Number | EC 606-853-1 |
| Gmelin Reference | 430033 |
| KEGG | C14370 |
| MeSH | D017209 |
| PubChem CID | 69752 |
| RTECS number | UY8225000 |
| UNII | J5U13U5A8F |
| UN number | UN2811 |
| Properties | |
| Chemical formula | C11H9NO |
| Molar mass | 193.22 g/mol |
| Appearance | Light yellow to yellow powder |
| Odor | aromatic |
| Density | 1.183 g/cm³ |
| Solubility in water | Insoluble |
| log P | 1.99 |
| Vapor pressure | 1.22E-3 mmHg at 25°C |
| Acidity (pKa) | 15.45 |
| Basicity (pKb) | 13.05 |
| Magnetic susceptibility (χ) | -60.91·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.613 |
| Viscosity | Viscosity: 0.952 cP (25°C) |
| Dipole moment | 3.95 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 270.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | Std enthalpy of formation (ΔfH⦵298) of 2-Benzoylpyrrole: **54.2 kJ/mol** |
| Std enthalpy of combustion (ΔcH⦵298) | –3907.7 kJ·mol⁻¹ |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | Precautionary statements: P261, P264, P271, P280, P302+P352, P305+P351+P338, P332+P313, P337+P313, P362+P364 |
| NFPA 704 (fire diamond) | 2-2-0 |
| Flash point | Flash point: 136.6 °C |
| Autoignition temperature | Autoignition temperature: 455°C |
| LD50 (median dose) | LD50 (median dose) of 2-Benzoylpyrrole: "894 mg/kg (rat, oral) |
| PEL (Permissible) | Not established |
| REL (Recommended) | 100 mg |
| Related compounds | |
| Related compounds |
2-Acetylpyrrole 1-Benzoylpyrrole 3-Benzoylpyrrole Pyrrole Benzoyl chloride 2-Formylpyrrole |