The story of 2-Aminothiazole Hydrochloride goes back to the era when scientists began exploring small heterocycles for their unexpected behavior in organic chemistry. In the middle of the twentieth century, researchers poked around thiazole cores, trying to tease out new routes for pharmaceuticals and dyes. German chemists liked how thiazoles kept popping up in natural compounds, and by adding an amine group, they landed on 2-Aminothiazole. Switching up the counterion to hydrochloride made it easier to handle in labs and gave a little boost in solubility. Gradually, these tweaks turned what could have been an obscure intermediate into a standard on chemical benches worldwide. Whenever a medicinal chemist flips through a catalog for a reliable nitrogen-sulfur heterocycle to toss into the next batch of syntheses, 2-Aminothiazole Hydrochloride ends up on a sticky note not because it’s flashy, but because it’s reliable and familiar.
Nobody asks for "2-Aminothiazole Hydrochloride" at the counter unless they have a clear goal in mind, but you’ll see it on order forms under alternate names like 2-Aminothiazolium chloride or 2-Thiazolylammonium chloride. Catalogs often list synonyms like “2-aminothiazolium hydrochloride” or abbreviate it as “2-AT·HCl.” Chemists learn to recognize the structure without blinking: a five-membered ring with both nitrogen and sulfur, an amine off the second carbon, balanced by a single chloride ion. It’s always white or off-white, tends to chunk up in the bottle, and feels grainier than it looks.
Working with 2-Aminothiazole Hydrochloride means counting on certain properties. Melting point hovers near 200°C, but it’ll decompose before melting cleanly; you’ll catch a sharp whiff if you push it too far on the hot plate. Water loves this salt, one reason it sees lab use. In ethanol or methanol, it dissolves after a minute or two of swirling, but it’s almost a stranger to non-polar solvents. The compound stands up to most bench conditions but breaks down under strong alkali, which limits certain reaction conditions. Keep it away from open air for days, or it’ll lump up from moisture, hinting at the balance between being handy and needing care.
Any real lab supply bottle comes with more than just the name. You get the chemical formula, C3H5N2S·HCl, and a purity figure—95% or above if you’re buying from a reputable company. Labels usually mention the lot number, expiry date, CAS number (2523-73-1), and sometimes heavy metal limits or drying requirements. Moisture content matters much more than most would think, since every milligram of water changes the stoichiometry for reactions downstream. Handling instructions warn against breathing the dust and urge you not to eat, drink, or smoke while pouring. These aren’t just CYA statements; a small slip can mix caustic tastes into any pizza break nearby.
Synthesis tends to start with thioformamide and α-haloketones, letting these churn together to close the ring. Tweak the conditions—more acid, less heat, longer reflux—and you get a crude thiazole. Introduce an amine in the right spot, usually ammonia or a simple amide, and you nudge the molecule toward 2-Aminothiazole. The last stage acidifies the batch, which binds the hydrochloride, pulls it out of solution as salt, and makes it ready for filtration. If you’ve followed the steps and dried the fluffy solid without it clumping up, consider it a successful batch. It’s more craftwork than many would admit; scale-up means figuring out how to keep yields above 80% without fouling glassware or sending sulfur fumes through the lab.
2-Aminothiazole Hydrochloride holds court in hundreds of coupling studies. The free amine on the thiazole ring reaches out for acyl groups, sulfonyl chlorides, or aromatic aldehydes, forming new bonds that show up in patent filings every year. Researchers prize this salt for easy substitutions at the 2-position and rapid formation of Schiff bases. Cheap, dependable, and reactive, the molecule supplies core fragments for kinase inhibitors, anti-fungal drugs, and agrochemical leads. Mix it with strong base, it sheds the hydrochloride and acts as a flexible nucleophile; use it in metal-catalyzed cross-coupling, and it adapts to new frameworks. Every modifications notebook in medchem sees a round or two with 2-Aminothiazole derivatives for hit expansion during lead optimization.
Even veterans pause before dumping a bag of 2-Aminothiazole Hydrochloride without gloves. Minor exposure causes irritation, especially if it catches an open cut or splashes the eyes. Bigger spills mean sorting out ventilation quick, since heat and moisture can coax noxious byproducts. Years of published MSDS sheets warn users to keep it below 25°C in sealed containers and always under a fume hood during reactions. In my own workspace, simple rules keep everyone safe: goggles on, skin covered, waste disposed of by sealing in polyethylene bags and sending to hazardous waste streams—never down the drain. Good labeling and routine safety checks do more to protect staff than any alarm ever will.
Drug discovery calls for libraries of compounds that probe biological targets, and 2-Aminothiazole Hydrochloride plays a reliable role as a core scaffold. Antimicrobial drug projects, especially tackling resistant bacteria and fungi, swarm with thiazole analogues thanks to their broad bioactivity. Dye synthesis taps the thiazole for vibrant yellows, while agricultural chemists rely on it when screening for new classes of pesticides or herbicides. If you browse scientific journals, you’ll see it mentioned in flame-retardant research, corrosion inhibitors, or even polymer additives. Every application leverages its reactivity and small footprint, stitching together complex molecules from a basic, affordable start.
Academic groups and pharmaceutical labs wage friendly competition on who synthesizes more diverse thiazole-based structures for biological screening. The most active work focuses now on anticancer and antiviral projects, with high-throughput screens often highlighting 2-Aminothiazole derivatives in their results. Medicinal chemists lean on the scaffold for its balance of metabolic stability and potential to pass drug-like filters. Researchers in material science twist the base structure, tying in unusual substituents to change color or conductivity. Patent filings stack up, but every published failure teaches others about where the limits fall for reactivity, solubility, or downstream biological handling.
Interest in thiazole toxicity grew after noticing some analogues hitchhiked into the brain or stuck around in the liver. Rodent studies show 2-Aminothiazole Hydrochloride poses moderate acute toxicity when ingested or inhaled, with reported LD50 values pointing to caution during weighing and mixing. Long runs with the compound strain liver function in model systems, but clinical crossover data looks limited—its main use lives in the test tube, not in the pill bottle. Environmental impact draws more scrutiny; improper disposal leads to bioaccumulation worries. Environmental health and safety officers urge labs to stick close to tried-and-true handling protocols and to weigh waste out before the end of each project.
2-Aminothiazole Hydrochloride doesn’t strike most chemists as revolutionary, but it quietly anchors next-generation molecular design. As machine learning and automated screening reshape the way drug leads emerge, small accessible fragments like this take on new roles in platform chemistry. Green chemistry trends push for new solvents and alternative preparation routes—cheaper, less wasteful, safer for everyone in the lab. Advances in click chemistry and photoredox catalysis hint at expanded use, as researchers try to attach thiazoles faster and with less fuss. As patent cliffs push pharmaceutical companies to explore new molecular scaffolds, this little salt keeps finding its way onto the bench, offering a mix of predictability and creative opportunity. It’s a workhorse compound—the sort that reminds everyone that progress in synthetic chemistry depends on sturdy building blocks as much as moonshot discoveries.
2-Aminothiazole hydrochloride pops up more often in research labs than it does in daily conversation. I discovered it during my own time working with anti-infective agents. Its value doesn’t just lie in theory. This compound has a real story to tell in the world of pharmaceuticals, agriculture, and chemical synthesis.
The real power of 2-aminothiazole hydrochloride can be found in drug development. Universities and pharmaceutical companies depend on small molecules like this when chasing the next breakthrough. The backbone of 2-aminothiazole finds its way into countless experimental drugs. Chemists build on this structure, swapping out groups and tweaking atoms, hoping to create something useful. The most impressive examples include potential antibiotics designed to knock back resistant infections. A 2022 report in Antimicrobial Agents and Chemotherapy drew links between thiazole-based drugs and improved efficacy against certain pathogens. Without molecules like 2-aminothiazole hydrochloride, much of this research would be dead in the water.
My grandfather worked on a grape farm, and I remember how frustrating fungal infections could get. Compounds built on the thiazole ring, including 2-aminothiazole hydrochloride, serve as anchors for fungicides and herbicides. They help chemists develop new blends that keep pests and diseases at bay. In this context, it isn’t about mass marketing or everyday use. Crop scientists are under pressure to solve fast-evolving problems, and they often lean on thiazole chemistry to do it. If resistance breaks out among weeds or blight takes hold, researchers sift through libraries of related molecules—hoping for the right fit. The agriculture sector depends on inventions sparked by small compounds with proven reactivity and versatility.
My early days in a chemical synthesis lab taught me the headache of building complex molecules from scratch. 2-Aminothiazole hydrochloride shines here, offering a flexible starting point for synthesis. It reacts with straightforward reagents and gives a predictable outcome, which means less troubleshooting for students and professionals alike. Asked to create a series of analogues? This compound handles substitution reactions with ease. As a result, it helps chemists save time and boost productivity. Journals in synthetic organic chemistry mention its role as a “building block” more times than anyone can count. Waste less time, get more results—that’s the rule in any busy lab.
Any time a new compound comes into play, safety steps up as a real issue. 2-Aminothiazole hydrochloride isn’t especially toxic compared to industrial solvents or heavy metals, but it demands respect. Gloves, fume hoods, and careful disposal should be routine in any setting. There’s increasing pressure on research groups and industry players to follow regulations about chemical waste. In my experience, tight safety checks cut down on accidents and speed up approval for new products. The global push for responsible research keeps growing. Scientists take chemicals like this seriously because public trust depends on it.
The next time someone points to a new drug or innovative crop protection formula, chances are the backstory includes compounds most people never hear about. 2-Aminothiazole hydrochloride bridges the gap between raw chemistry and useful technology. Its versatility keeps it in play for those building new solutions to pressing problems—both on the farm and in the pharmacy. By supporting new research and safer practices, chemists keep pushing boundaries, and that’s where this compound leaves its mark.
I remember the first time I came across 2-Aminothiazole Hydrochloride in the lab. The name might sound intimidating, but it’s just a compound with the formula C3H5N2S·HCl. The molecular weight lands at 152.61 g/mol. These details go way beyond trivia. They’re what researchers and manufacturers anchor on for dosing, synthesis, and quality control. Trust me, getting a formula wrong can’t just mess up an experiment — it can throw off entire production batches.
Basic as it sounds, knowing a chemical’s formula means you can predict reactions, calculate yields, and even check purity with confidence. I’ve seen what happens when chemists overlook these numbers: wasted time, wasted money, sometimes safety risks that no one wants to talk about. 2-Aminothiazole Hydrochloride, despite its small size, works as a key piece in pharmaceutical syntheses and other applications. Its hydrochloride salt bumps up its water solubility, making it a lot easier to handle in reactions. That solubility directly links back to the need for proper calculations—too little or too much changes everything, from solubility to reactivity.
In my early days as a research assistant, 2-Aminothiazole Hydrochloride showed up in several antimicrobial projects and synthetic routes for some high-profile drug candidates. No one lacked respect for its reactivity. Step into a pharmaceutical lab and you’ll hear folks talk about needing compounds that react on cue. That comes down to knowing the exact molecular formula and molecular weight. A compound like this doesn’t just sit on a shelf. People weigh it, dissolve it, react it, and filter it — precision means fewer mistakes, tighter results, and a safer lab.
I’ve seen a lot of students overlook the basics, thinking the formula or molecular weight isn’t a big deal. That mistake costs more than time. Incorrect data leads to incorrect mixes. For pharmaceuticals, that means failed quality tests or worse, patient risk. Lab veterans know these risks and double-check batch certificates, chemical labels, and even run quick calculations by hand. Working with any hydrochloride salt, eyes sting if something spills, so having clear labeling — including the formula and molecular weight — keeps everyone safer. The paperwork is more than bureaucracy. Sourcing traceable compounds with a guaranteed formula ensures nothing comes back to bite you later.
Most chemists use digital tools these days to check formulas and molecular weights. The old days of thick CRC handbooks sitting out on benches may be gone, but accuracy still lives at the core of good science. Lab training goes over these basics constantly. We quiz new folks, run mock reactions, and find slip-ups before anything scales up. Best practices aren’t a luxury — they’re how real labs avoid disasters.
The next time someone glosses past the formula or weight of a chemical like 2-Aminothiazole Hydrochloride, I think back to spilled beakers, botched syntheses, or even endless explanations during audits. A little care upfront saves a lot of trouble down the road. Precision isn’t glamorous, but it keeps us honest. For anyone in chemistry, that’s everything.
Working with chemical compounds in a lab isn’t just about fancy glassware and obscure labels. It’s about keeping things safe and making sure tomorrow’s results look as reliable as today’s. I’ve handled a lot of chemicals over the years, and 2-Aminothiazole Hydrochloride doesn’t stand out as something you can just leave on a shelf. It absorbs moisture from the air, it degrades if exposed to light for too long, and it reacts with substances most folks wouldn’t think twice about.
For starters, I’ll never forget the morning we opened a container and found the compound had clumped up. Someone left it near the window. The lesson stuck: this is not a powder meant for room temperature or sunlight. It expects a dry spot, away from direct light and humidity. A sealed, amber bottle provides a basic line of defense. Humidity seeps in without much warning—desiccators, those simple airtight boxes with drying agents, come through every time. Lab fridges also give an extra layer of confidence if things heat up, especially during summer.
In one of my early jobs, an underfunded research group tried cutting corners—regular plastic jars, shelves exposed to daylight, and a cluttered workspace. After a month, half the chemicals—including our 2-Aminothiazole Hydrochloride—became useless. Moisture turned the dry powder into unusable mush. Replacement cost more than we’d budgeted for the entire quarter. For smaller labs and startups, that’s not just annoying—it’s the kind of mistake that stalls projects.
Nobody wants their project derailed by a mishap. I’ve seen careless storage spark more problems than actual experiments: explosions, unknown vapors, ruined work. It pays to keep the container tightly closed, out of the sunlight, in a room with good ventilation. In some labs, policies spell it all out in binders, but habits matter more than paperwork. I always make sure bottles go back into the right space straight after use, and labels are clear. Sometimes, extra reminders—like a sticker or note—keep things where they belong.
At the end of the day, real solutions start simple: clear labeling, unbroken seals, and regular checks. Storing 2-Aminothiazole Hydrochloride in a desiccator or drybox, in a refrigerator set to about 2–8 °C, keeps it safe for months. Dry hands and dry tools prevent unexpected reactions or clumping. For bulk storage, splitting the compound into smaller vials makes life easier—no need to open the main container every time. This way, if something does go wrong, only a small batch gets ruined.
Chemical storage may not sound glamorous, but the costs of cutting corners add up fast. Good habits mean less stress, fewer emergencies, and a whole lot more peace of mind when working late at night. Investing in decent storage gear pays back in saved time, safer workspaces, and fewer ruined materials. After years in labs big and small, it’s clear: storing compounds like 2-Aminothiazole Hydrochloride safely starts with treating each container like tomorrow’s success depends on it—because it usually does.
Stepping into any lab with chemical compounds in hand, folks should have safety on their minds. My own days working around chemicals taught me the value of foresight. The tiniest mistake – a forgotten glove, a missed step – can easily turn routine work into a hazard. If you ever come across 2-Aminothiazole Hydrochloride, it’s not the sort of material to treat lightly. The way this compound behaves, the kind of risks it brings, and the best approach to managing those risks, all call for a bit of know-how and practical sense.
2-Aminothiazole Hydrochloride looks like another fine powder in a bottle, but I’ve learned appearances rarely tell the whole story. Dust from this compound can irritate the nose, lungs, and eyes without much warning. Breathing it in over time may not seem like a big deal at first, but the build-up in a poorly ventilated room can turn into a sneaky health problem. Getting the powder on your skin can cause redness or even rashes, especially after a day’s exposure.
Spills seem harmless at a glance, yet the chemical’s fine texture lets it scatter easily, slipping through the smallest cracks. Laboratories with tons of activity see enough residue spread around, creating risk where none should exist. I’ve cleaned enough bench tops to realize just how fast one stray beaker can mess things up. On top of that, the hydrochloride version likes water, and that sometimes means extra caution keeping things dry.
I’ve seen new lab workers groan at long safety checklists, but picking up a few habits pays off in the long run. Gloves – good, solid nitrile or latex ones – stand between skin and the compound. Fitted eye protection keeps splashes at bay, and working inside a fume hood gives lungs a rest from stray dust. These steps feel routine after a week or two, but they make all the difference during close calls.
It’s crucial to pay attention to labels, and never trust a half-torn sticker. Storing 2-Aminothiazole Hydrochloride means keeping it in a tight container away from food, coffee cups, or any snack people like to sneak into the lab. Mixing chemicals without proper knowledge has caught even experienced hands off guard, so reading chemical compatibility charts always helps before reaching for another bottle.
No matter how careful, spills sometimes happen. Sweeping up powder instead of blowing it around makes a world of difference. A damp disposable towel traps dust. My mentor used to line every clean-up kit with extra gloves, towels, and a clear set of instructions. Washing hands before stepping away from the bench should become second nature.
Eye wash stations and showers aren’t just warning signs on a poster: they must work and stay within easy reach. I’ve done surprise checks on these stations, and sometimes found eyewash bottles stuck shut from disuse. Testing them weekly kept peace of mind. Inhalation accidents need fresh air right away, and serious exposure calls for calling in a medical professional pronto, not just hoping symptoms pass by.
Training matters as much as gear. Everyone from new interns to regulars ought to talk through risks, ask questions, and hold each other accountable. The moment folks get used to skipping steps, that’s the start of real trouble. Building a culture of speaking up early can stop accidents before they snowball. 2-Aminothiazole Hydrochloride doesn’t ask for fear, but it reminds us all to keep respect and planning at the center of safe science. Years of careful work stack up to create habits that last, keeping hands, eyes, and airways out of harm’s way for everyone on the team.
Buyers looking for 2-aminothiazole hydrochloride often run into the tricky question of grades and purities. This compound, with its odd little sulfur and nitrogen ring, finds use in all sorts of labs—drug research, agricultural experiments, and dyes, to name a few. But not every project calls for the same batch, and certainly not every wallet matches the price tags on the top-shelf grades.
Grades usually come down to two ideas: what's in it and what's left out. If a researcher wants to use this chemical as a starting block for a new antibiotic, every stray speck can matter. In big pharma or biotech, trace metals or leftover solvents from manufacturing can quietly ruin a week’s work. For those jobs, folks lean on high-purity grades—sometimes over 99%. These can cost more, but it beats watching an experiment fizzle because of an impurity.
Not everyone needs that. Someone making dyes or testing ideas that won't see a hospital might go with technical or laboratory grade, which doesn’t reach for the same purity. Results can still shine, costs stay down, and suppliers move more inventory. In my early chemistry days in graduate school, rule number one was check the grade twice before checking out and never pay for more than you use. One of my mentors joked that wasting money on high-grade solvents was like filling your car with rocket fuel to run errands.
Purity isn’t always measured the same way. You might see “analytical grade,” “laboratory grade,” or “reagent grade” on product sheets. Some countries lean on standards written by organizations like ACS (American Chemical Society) or European Pharmacopeia. But these labels can sometimes blur in the real world, where one producer’s “reagent grade” edges close to another’s “analytical.” Real chemistry happens in labs, and so do the phone calls: asking for certificates of analysis, digging into batch numbers, running your own spot checks.
Quality varies, and it comes down to trust and verification. Some researchers have been burned by “surprise” contaminants—you buy what you thought was pure, then find an unknown peak in an HPLC trace. I remember standing over a spectrometer, staring at a weird bump, thinking, “There goes three days.” Cleaning up those mistakes wastes time and burns through funds, which nobody likes.
One solution starts with education—knowing exactly what your experiment can handle and how much risk you’re willing to allow. If the goal is drug synthesis or critical trials, there’s wisdom in shelling out for high-purity lots from trusted suppliers, and always checking for up-to-date paperwork. For high-volume or less sensitive industrial uses, technical grade can make sense, but it pays to sample and test every new order, since batches can drift over time.
If a supplier can't be clear about grade or can't show a proper analysis, that’s a red flag. In my own work, keeping a record of bad or unreliable lots helped convince procurement to stay sharp and push back on vendors. Forward-thinking labs, especially in universities, sometimes set up club buying or share test data among departments to keep everyone aware of which batches deliver.
At the end of the day, purity shapes results. 2-aminothiazole hydrochloride sits in a long line of chemicals where small differences matter. Keeping tabs on what lands on the bench saves labs from expensive headaches and keeps research moving in the right direction. For companies and researchers alike, staying informed creates better science and smarter spending.
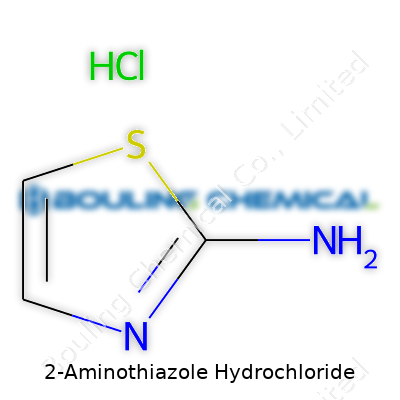
| Names | |
| Preferred IUPAC name | 2-Aminothiazol-1-ium chloride |
| Other names |
2-Aminothiazole hydrochloride 2-Amino-1,3-thiazole hydrochloride 2-Thiazolamine hydrochloride |
| Pronunciation | /tuː əˌmiːnoʊ θaɪˈæzoʊl haɪˌdrɒklaɪˈraɪd/ |
| Identifiers | |
| CAS Number | 1640-39-7 |
| 3D model (JSmol) | `JSME 3D Model String for 2-Aminothiazole Hydrochloride:` ``` Cl.Nc1nccs1 ``` |
| Beilstein Reference | 127878 |
| ChEBI | CHEBI:38274 |
| ChEMBL | CHEMBL226679 |
| ChemSpider | 145215 |
| DrugBank | DB08447 |
| ECHA InfoCard | echa:100.035.232 |
| EC Number | 29809-40-9 |
| Gmelin Reference | 72497 |
| KEGG | C14129 |
| MeSH | D000900 |
| PubChem CID | 22034043 |
| RTECS number | XZ3850000 |
| UNII | 3F592FI94G |
| UN number | 2811 |
| Properties | |
| Chemical formula | C3H5ClN2S |
| Molar mass | 134.60 g/mol |
| Appearance | White to off-white solid |
| Odor | Odorless |
| Density | 1.38 g/cm3 |
| Solubility in water | Soluble |
| log P | -2.3 |
| Acidity (pKa) | 6.0 |
| Basicity (pKb) | 7.98 |
| Magnetic susceptibility (χ) | -63.5×10-6 cm³/mol |
| Dipole moment | 3.99 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 196 J·mol⁻¹·K⁻¹ |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P273, P280, P302+P352, P305+P351+P338, P312, P337+P313, P362+P364 |
| NFPA 704 (fire diamond) | 2-(1)-0 W |
| LD50 (median dose) | LD50 (median dose): Mouse oral 920 mg/kg |
| NIOSH | Not Listed |
| PEL (Permissible) | Not established |
| REL (Recommended) | REL (Recommended): 2-8°C |
| Related compounds | |
| Related compounds |
2-Aminothiazole Thiazole Benzothiazole 2-Mercaptothiazole Thiazole-4-carboxamide |