Scientists started to notice the thiazole ring system in the early twentieth century. This backbone caught attention for its presence in bioactive molecules, especially in antibiotics and enzyme inhibitors. As medicinal chemistry pushed ahead, researchers synthesized derivatives like 2-Aminothiazol-4-acetic acid, looking to harness its potential in drug development. Over the years, academic groups and pharmaceutical companies recognized how modifications to the thiazole nucleus could produce new treatments, especially once penicillin analogs and cephalosporins introduced these motifs into the medical mainstream.
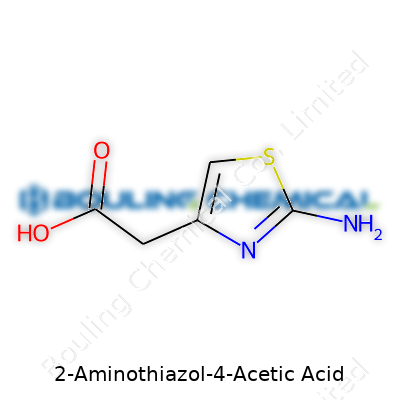
2-Aminothiazol-4-acetic acid, often found in laboratories with the formula C5H6N2O2S, is a white to off-white crystalline powder. Folks who work with this material recognize it as a building block in both organic synthesis and pharmaceutical research. Its reliable structure — a thiazole ring fused to a terminal amino group and acetic acid side chain — provides versatility. Chemists often keep this compound on hand when crafting new antibiotics, picking it for its ability to interact with a range of other chemicals and serve as a precursor in the creation of more complex drugs.
At room temperature, 2-Aminothiazol-4-acetic acid presents as a solid. Its melting point usually hovers around 220°C, reflecting a stable structure compared to other amino acids. The molecule’s solubility falls in line with its moderate polarity, dissolving better in water and alcohols than nonpolar solvents. The amino group gives it basic character, while the carboxylic acid provides acidity, making it amphoteric and handy for buffer and salt formation. Its crystalline shape and reliable purity help both in benchwork and in scaled-up manufacturing.
Producers list the purity above 98%. On chemist’s shelves, labeling always specifies both batch and lot number, molecular weight (158.18 g/mol), and storage conditions. Stability remains highest in tightly sealed containers, out of direct light, usually at room temperature. Labels also carry the chemical structure and recommended chemical codes following IUPAC guidance. Anyone handling the product can quickly identify hazard pictograms if applicable and note the recommended personal protective equipment. This clarity keeps workflow smooth and maintains safety standards in any lab or factory environment.
Synthesis begins with thiosemicarbazide, reacting with α-haloacetic acid in the presence of sodium acetate. Heating this mixture, the cyclization forms the thiazole ring, and simple workup yields pure 2-Aminothiazol-4-acetic acid after crystallization. Different labs sometimes optimize yields by tweaking reaction times and solvents, but the core chemistry stays the same. This straightforward preparation makes the compound accessible even to smaller research outfits.
The molecule’s value shows up in its reactivity. Chemists rely on the amino group for acylation, sulfonation, and alkylation, each offering access to other derivatives. The carboxylic acid opens the door for amidation or esterification, providing pathways that feed directly into the development of new beta-lactam antibiotics. In research settings, teams introduce protecting groups to control selectivity during multi-step syntheses. The thiazole ring itself can serve as a platform for substitution, leading to analogs with distinct pharmacological activity.
Scientists and suppliers alike use names such as 2-ATAA, 4-Acetic acid-2-aminothiazole, and 2-Amino-4-thiazoleacetic acid. Databases often list it under CAS number 2212-42-4. In pharmaceutical contexts, vendors may refer to it simply as a thiazole-acetic acid derivative. The variety in chemical naming underscores the compound’s history and widespread use across industries.
While handling, gloves, goggles, and lab coats make sense to reduce any chance of skin or eye contact. Even though the general toxicity is reported low, dust inhalation or accidental ingestion calls for caution. Safety data sheets highlight risks associated with heating or mixing with strong bases or acids, and suggest local exhaust ventilation to keep exposure in check. Laboratory workers and production staff receive clear training on spill management and disposal, following both regulatory and institutional guidance.
2-Aminothiazol-4-acetic acid thrives as an intermediate in medicinal chemistry. Pharmaceutical teams use it for assembling cephalosporins, a major class of antibiotics active against resistant bacterial strains. Some plants use it to produce herbicides and fine chemicals, relying on its ready reactivity and predictability. Research groups, seeking new enzyme inhibitors or anti-inflammatory drugs, often pick it as a starting point. Its flexible structure and reactivity mean it finds a role in labs around the globe, from academic benches to industry R&D centers.
Labs around the world use 2-Aminothiazol-4-acetic acid to discover new pharmaceutical leads. Some teams try tweaking the molecule’s structure to boost activity or lower side effects in new drugs. Combinatorial chemistry practices often pull it into large-scale screening libraries. Its commercial availability, affordable cost, and manageable synthesis keep it in steady demand among biotech startups and contract research organizations. As funding moves toward antibiotic innovation, this building block stands near the center of many project blueprints.
Most data so far suggest low acute toxicity. Animal testing at moderate doses shows rare adverse effects, and environmental persistence does not reach high levels. Regulatory agencies set workplace exposure guidelines based on its reactivity and irritation potential, not long-term organ damage. Ongoing studies probe how derivatives may behave in the body and whether specific formulations could trigger allergic reactions or metabolic issues. Researchers understand the need for robust in vitro and in vivo data to ensure new drugs meet modern safety standards.
Advances in green chemistry influence how companies look at updating 2-Aminothiazol-4-acetic acid synthesis. Sustainable routes, such as enzyme-catalyzed processes or recyclable solvents, look promising. In the clinic, new resistance patterns drive a return to thiazole-based antibiotics, shining a bright light on this core structure. Besides healthcare, companies see opportunity in crop protection and veterinary medicine, each demanding novel molecules to keep pace with changing needs. Open science and growing data transparency help teams worldwide collaborate on new scaffolds rooted in 2-Aminothiazol-4-acetic acid, creating hope for solutions to both current and future challenges in medicine and industry.
2-Aminothiazol-4-acetic acid carries the chemical formula C5H6N2O2S. This compound pulls together five carbon atoms, six hydrogens, two nitrogens, two oxygens, and a sulfur atom. Each piece holds its place for a reason—chemists love to point out the practical value of such details, right down to where that sulfur atom sets the thiazole ring apart from most amino acids.
In the world of antibiotics, researchers often come across 2-aminothiazole rings within many breakthrough molecules. Thiazole-based structures form a backbone in drugs like cefotaxime and ceftriaxone—both advanced cephalosporins tackling infections that used to spell real trouble in hospitals. The acetic acid group helps the molecule interact with enzymes and proteins. Every atom in this formula plays its role in making the compound more useful or less toxic in real applications.
As someone who's handled organic syntheses in real lab settings, certain formulas stick in your mind because they drive your research forward or solve stubborn issues. Pouring over reaction mixtures late at night, the structure of 2-Aminothiazol-4-acetic acid often comes up when you try to build analogues for better activity or solubility. Chemists appreciate formulas not just for their neatness but for where they take you—a step away from the unknown and toward a new solution for real diseases.
Plenty of students get hung up on memorization, yet working scientists focus on application. In the chemistry of antibiotics, the aminothiazole group helps break through bacterial defenses that resist older drugs. By knowing which atom sits where, pharmaceutical developers tune the structure to make the medicine stronger and longer-lasting. The precise formula means researchers don’t just guess—the journey from petri dishes to pharmacy shelves needs clear, reliable information.
Getting your head around the formula matters for more than chemical trivia. During synthesis, it’s easy to imagine you’ve made the right molecule, but analytical methods—NMR, IR, sometimes mass spectrometry—bring reality into sharp focus. Even a missing hydrogen or mislocated oxygen changes everything. The rise of antibiotic resistance around the world calls for new molecules, better screening, and accurate data.
Institutions often support young chemists in learning about these core formulas. Hands-on workshops, real-time analysis, and peer collaboration matter more than rote theory. Strong mentorship leads many to see the application in public health and not just on paper.
From my own experience and the evidence in the field, support for open research, better data sharing, and practical training in chemical analysis makes a big difference. Partnerships across disciplines—pulling in biologists, pharmacists, and computational experts—boost the chances of finding new uses for structures like 2-Aminothiazol-4-acetic acid. The journey from bench to bedside always starts with a solid grasp of both the formula and the people who dig into its details.
Some chemicals end up in just about every corner of the science world, and 2-Aminothiazol-4-Acetic Acid is a good example. This compound builds bridges between basic research and the medicines we use daily. At the bench, chemists rely on it as a powerful building block, bringing its nitrogen and sulfur ring into reactions that create much more complex molecules. Its structure gives scientists room to explore efforts against stubborn infections and chronic diseases, and that’s what makes it such a standout.
New drugs don’t just show up overnight, and the road starts with chemical building blocks like this one. In my time following the pharmaceutical field, the thiazole ring—part of this molecule—keeps popping up in life-saving drugs. Probably the most recognized are antibiotics. Take cefotaxime, used for years in hospitals, which draws directly from this type of chemistry. Medicinal researchers saw that adding a thiazole ring, especially the aminothiazole found here, made antibiotics work harder against tough bacteria. This small tweak gave doctors new tools when old antibiotics started failing. That’s the kind of direct, practical impact you see in real hospitals.
Beyond bacteria, researchers test 2-Aminothiazol-4-Acetic Acid as a starting spot for anti-inflammatory, antiviral, and even anticancer agents. Journals tell the story: new derivatives built from this compound often land in preclinical studies because the core ring can be tuned in all sorts of inventive ways. It’s one of those rare ingredients where a minor chemical shift can mean the difference between a weak molecule and a drug that changes someone’s life.
No surprise, chemistry doesn’t live only in the hospitals. I’ve talked to friends working in crop protection and agricultural chemistry; they’re always on the hunt for safer, more effective ways to protect harvests. 2-Aminothiazol-4-Acetic Acid feeds into their work, too. The thiazole backbone pops up in modern herbicides and fungicides, helping farmers battle pests that can wipe out entire fields. Some research teams also use this compound early in projects, seeing potential for safer pesticides that protect crops without putting people or pollinators in harm’s way. With food safety and environmental concerns only growing, hitting a balance here is critical.
This compound’s wide reach brings both opportunity and responsibility. Academic teams want to invent new medicines, but they need to know the risks along the way. All that handling in the lab means proper ventilation, gloves, and training become just as important as curiosity. Missteps in handling can set back months of work, or worse, pose health hazards to the people involved. Large pharmaceutical companies and universities both work with strict guidelines—the kind set by the Occupational Safety and Health Administration and similar bodies worldwide. Beyond labs, regulators also want to see environmental testing, especially if these chemicals land in agriculture.
With antibiotic resistance on the rise, the world needs creative molecules more than ever. Investment in smart, targeted chemistry using compounds like 2-Aminothiazol-4-Acetic Acid gives hope that the next generation of drugs can outpace disease. Collaboration across industries—from pharma to agriculture—offers the best shot at safe, innovative progress. Making the most of this unique building block means investing in both discovery and safety, pushing science toward solutions that matter in the real world.
2-Aminothiazol-4-acetic acid, a commonly used intermediate in pharmaceutical synthesis, shows sensitivity to both moisture and light. This property stands out because even trace exposure can alter its performance in later stages of drug making. Anyone who has spent time in a laboratory knows just how crucial it is to keep chemicals in the right state—one lapse, and the next batch of research work risks contamination or unexpected results. The hassle of troubleshooting degraded product isn’t just annoying; it’s expensive and often wastes valuable time.
Based on supplier guidelines and chemical safety data sheets, the best way to store 2-Aminothiazol-4-acetic acid involves keeping it in a tightly sealed amber bottle. Place this container in a cool, dry spot, away from direct sunlight. Temperatures between 2°C and 8°C—the average refrigerator range in labs—help prolong the compound’s usable life. Excessive heat or humidity shortens shelf life rapidly, and there’s more risk of impurity formation that nobody wants to see show up on an HPLC chromatogram.
Many labs fall into the trap of dropping jars on any shelf with free space. That’s a recipe for disaster with delicate compounds. At one biotech startup, a mislabeled shelf next to a steam radiator led to a whole shipment spoiling—three weeks of procurement lost, and a round of finger-pointing nobody learned from fast enough. Clear labeling, temperature logging, and moisture indicator packets cut down on these avoidable mistakes. Chemical suppliers warn about hydroxyl or amine impurities showing up after improper storage, which can ruin both research runs and manufacturing output.
Safe storage helps protect both people and science. Handling 2-Aminothiazol-4-acetic acid with respect guards against unnecessary exposure; nobody loves glove changes or surprise headaches from mishandled powder. A solid routine with chemical storage also helps meet regulatory expectations. Audits from the FDA or local safety officers often boil down to simple questions: Are chemicals labeled and protected from the elements? Have staff kept temperature records? I’ve seen more than one project delayed for something as basic as “room temperature” chemicals sitting under faulty HVAC.
A reliable setup—proper refrigeration, regular checks, and clear records—keeps the guessing games out of the process. That routine also keeps everyone safer. Even outside direct pharmaceutical use, reproducibility relies on using clean, uncontaminated reagents. A few degrees too warm, a lid left off, or light sneaking in through a window can be the difference between an experiment’s success and weeks of wasted effort.
To build a strong routine, take time to review the material’s Safety Data Sheet. Invest in robust refrigeration with alarms for temperature swings. Clearly marked, light-proof containers make retrieval easier and safer. Assign staff to check expiry dates and conditions every month. Get used to adding silica gel or desiccants to limit humidity. Train new lab members to spot the signs of spoilage, like color changes or clumping. These routines lift up not just productivity, but also team morale. Fewer spoiled chemicals mean better results and less frustration.
Day-to-day, these habits mean the difference between data you can trust and results you keep having to explain. For anyone working with research chemicals—students, scientists, production techs—good storage represents more than habit. It’s a mark of respect for the work itself.
Anyone who works with organic synthesis and medicinal chemistry runs into 2-Aminothiazol-4-acetic acid at some point. Sitting on a bench in a chemistry lab, staring at white or off-white powders, every researcher questions: is this material pure enough for my next reaction?
Chemical purity tells you how much of the material in your vial comes from what the label promises and how much comes from anything else—the leftovers, the byproducts, the dust that ruins a reaction or skews a set of test results. If you’re making pharmaceuticals, 99% pure still can mean up to 1% impurities, and that trace amount throws a wrench into a process designed to be clean and predictable. From my own experience with chromatography columns fouling up, purity shortfalls create real headaches, not hypothetical risks.
The standard for 2-Aminothiazol-4-acetic acid hovers at 98% or above, according to big-name suppliers and research chemical catalogs. Labs that make fine chemicals, especially for pre-clinical research or synthesis of drug intermediates, often call 98%-99% the cut-off. The leftover 1%-2% includes related thiazole-based structures, trace acids, moisture, and sometimes small bits of solvents caught during crystallization.
Specifications don’t end at a single purity number. Quality assurance teams demand a whole menu: melting point checks (usually around 162-166°C), a clear TLC test (one sharp spot at the appropriate Rf), and no more than 0.5% water by Karl Fischer titration. High-Performance Liquid Chromatography (HPLC) results matter here, since they show the real chemical profile. When buying batches for scale-up, labs request HPLC chromatograms from vendors—just to spot sneaky impurities before they show up in the NMR spectra downstream.
In pharma and agrochemical research, the purity of a starting material like 2-Aminothiazol-4-acetic acid directly shapes the purity of the final molecules. Most side products in bench chemistry stem from unwanted contaminants in the input, and nobody wants to hunt down a spectral ghost only to realize it entered the mix on day one. This isn’t just academic—regulatory guidelines (like ICH Q3A/B for pharma or ISO 17025 for lab standards) call for documentation of impurities above certain thresholds.
Sometimes getting the last 1% of purity isn’t worth the trouble or expense. There’s a trade-off between budget and synthetic success. In a big-budget drug screen, you’ll find no compromise—only 99%+ material passes. In early-stage research, 97% or 98% convinces many teams they can press ahead. Every chemist makes calls based on what their next step demands. More complex syntheses feel the pain sooner and demand better material up front.
Solving purity limits starts with transparent data. Manufacturers do well to share not just the percentage purity but full analyses: NMR, MS, and trace metals data. End-users ought to demand those proofs before batches come through the door. Better batch records and routine spot-checking with in-house spectroscopy keep projects safe from unexpected hiccups.
As methods like preparative HPLC or advanced crystallization filter down to more suppliers, more labs will have access to better quality—a win for tight budgets and smoother chemistry alike.
References:Anyone who has spent real time in a laboratory has brushed up against the long list of substances that demand respect. 2-Aminothiazol-4-acetic acid pops up in organic synthesis and research work, especially around pharmaceutical projects. Colleagues might find it shelved next to a dozen structurally similar compounds, each carrying safety quirks. Too often in academic labs, bottles lose labels, and new faces don’t realize small vials should get more than a casual glance.
This molecule doesn’t stand out as a major villain like cyanide salts or mercury, but it lands squarely on the list of chemicals smart researchers treat with caution. In published safety data, it shows low to moderate acute toxicity through ingestion and inhalation. Eyes and skin can show irritation on contact. The white crystalline powder won’t leap out or react explosively, but that bit of caution can keep an ordinary day from turning into a scramble for an eyewash.
Without protective eyewear or gloves, the story gets familiar: a splash during weighing, a bit of carelessness. Inhalation of dust can trigger mouth, throat, and nose discomfort. Lab veterans remember coughing fits, headaches, or irritations from less “notorious” chemicals. There’s no badge for pushing through stinging hands or itchy skin, and years in the field have shown how even moderate irritants can turn memorable if respect fades.
Ingestion, accidental or otherwise, risks more than just mild gut irritation. Researchers always rest easier after checking the Safety Data Sheet before popping open a new container, because trace exposures build up, and acute events don’t care if the stuff “isn’t as bad as” something else.
Standard lab protocols—dedicated ventilation, nitrile gloves, splash goggles—never feel like overkill. Every training orientation, some grad student asks if they really need all this for “minor” reagents. My answer: the guy who tossed aside his safety goggles during a short experiment spent hours rinsing out his eyes, and the paperwork lasted longer.
Solid practices include weighing powders in a fume hood, swapping out gloves that pick up visible dust, and labeling bottles clearly, even if time feels tight. Fume hoods aren’t only for volatile solvents—fine powders used day after day drift up, and most people overestimate how much escapes the weighing boat.
For spills, daily clean-up with damp paper towels followed by industrial-strength wipes beats letting residues build up in the corners. Staff meetings in well-run research groups stress the same thing: nothing wastes research time faster than an avoidable safety scare. Those who store chemicals in dry, well-ventilated spaces control both hazards and mess. It pays to keep the Safety Data Sheet handy, not stuffed in a forgotten folder.
Labs staffed with veterans know that spelling out risks for every compound isn’t about paranoia. Regulations serve as a floor, not a ceiling. It helps to treat everything new as hazardous until data and practice show otherwise. Even low-to-moderate risks accumulate, especially in places where turnover runs high and experience sometimes walks out the door.
No one expects 2-aminothiazol-4-acetic acid to spark news headlines, but steady, mindful work and honest risk conversations make sure stories around this bench remain boring. Most accidents stem not from wild chemistry, but from skipping steps everyone knows. Good habits serve everyone, from the newest undergrad to the most battle-scarred PI.
| Names | |
| Preferred IUPAC name | 2-amino-1,3-thiazole-4-acetic acid |
| Other names |
2-Amino-4-thiazoleacetic acid 2-Amino-4-thiazolylacetic acid 2-Amino-thiazole-4-acetic acid 2-Amino-1,3-thiazole-4-acetic acid ATAA NSC 5866 |
| Pronunciation | /tuː əˈmiːnoʊ θaɪˈæzɒl fɔːr əˈsiːtɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | [29649-42-9] |
| 3D model (JSmol) | `3d:12 C1N=C(S1)CC(=O)O` |
| Beilstein Reference | 3198703 |
| ChEBI | CHEBI:85169 |
| ChEMBL | CHEMBL1232742 |
| ChemSpider | 101828 |
| DrugBank | DB08347 |
| ECHA InfoCard | 100.049.601 |
| EC Number | 872-579-7 |
| Gmelin Reference | 8779.13 |
| KEGG | C14416 |
| MeSH | D000869 |
| PubChem CID | 123158 |
| RTECS number | AJ7875000 |
| UNII | P5043F38X0 |
| UN number | UN3335 |
| Properties | |
| Chemical formula | C5H6N2O2S |
| Molar mass | 158.19 g/mol |
| Appearance | White to off-white solid |
| Odor | Odorless |
| Density | 1.54 g/cm³ |
| Solubility in water | Slightly soluble in water |
| log P | -1.19 |
| Vapor pressure | 0.0000175 mmHg at 25°C |
| Acidity (pKa) | 2.90 |
| Basicity (pKb) | pKb = 11.09 |
| Magnetic susceptibility (χ) | -56.0 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.650 |
| Dipole moment | 4.51 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 178.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -683.7 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. |
| Precautionary statements | P264, P261, P271, P280, P302+P352, P304+P340, P312, P332+P313, P362+P364 |
| Flash point | Flash point: 242.9 °C |
| Lethal dose or concentration | LD50 Oral Rat 590 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 > 3120 mg/kg |
| NIOSH | NA7900000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.5 mg/m³ |
| IDLH (Immediate danger) | NIOSH: Not Listed |
| Related compounds | |
| Related compounds |
Thiazole 2-Aminothiazole Thiazole-4-acetic acid 2-(4-thiazolyl)acetic acid 2-Aminothiazole-4-carboxylic acid |