Chemists in the early 20th century started poking around the thiazole ring system to see what they could drag out of it. By the 1930s, folks noticed that dropping a nitro and an amino group onto thiazole made interesting new compounds, including 2-Amino-5-Nitrothiazole. At a time when infectious diseases loomed large and antibiotics were in high demand, labs hustled to push beyond sulfa drugs, trying new modifications to existing scaffolds. In those smoky labs and cramped offices, from European dye firms to American research departments, a host of medicinal candidates emerged, with 2-Amino-5-Nitrothiazole catching immediate attention for its potential in antibacterial research. Over decades, with society's shifting focus from infectious disease to cancer, attention on this compound waxed and waned, but it earned a spot as a reliable intermediate in both scientific literature and industry.
2-Amino-5-Nitrothiazole doesn’t tend to grab headlines or anchor pharmaceutical pitches, but anyone who has knocked around a synthetic chemistry lab probably recognizes its sharp yellow tint and distinctive whiff. Firms catalog it as a building block, with its nitro and amino groups dangling out, almost inviting further reaction. Combinatorial chemists shuffle it into libraries searching for new biological activity, while process chemists stash it on their shelves as a go-to for thiazole elaboration. Not some workhorse like acetic acid, but far from obscure, it remains one of those specialty chemicals—storied, approachable, and useful.
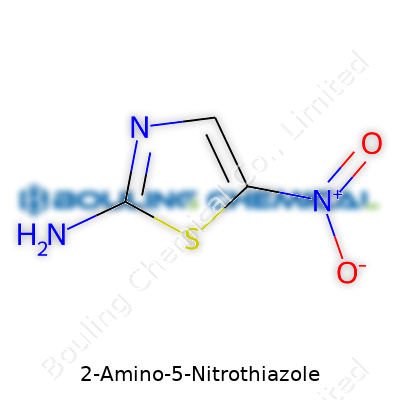
The physical side of 2-Amino-5-Nitrothiazole tends to be straightforward: a yellow crystalline powder, powdery to the touch and quick to stain gloves. Melting hovers around 204-207°C, which means it won’t vaporize in a typical workspace but has enough stability for basic thermal manipulations. Solubility gives a mixed bag: water handles it only slowly, but it goes down easier in hot water, dilute acids, and polar organic solvents like DMF. The structure (C3H3N3O2S, molar mass about 145 g/mol) packs the electron-hungry nitro group at the five spot, cranking up the compound’s reactivity and cutting its straightforward thiazole aroma. The combination of the electron-rich amino group and the strong nitro at opposite ends of the ring primes this little molecule for more sophisticated synthetic exploits.
Bags of 2-Amino-5-Nitrothiazole typically list a purity north of 98%. Real lab rats look for moisture content, specify maximum allowable ash, and ask for a HPLC or TLC analysis just to confirm integrity. Labels read like a litany: CAS number 121-66-4, batch number, manufacture and expiry dates, net weight, handling pictograms, and hazard warnings. The color, crystal habit, and solubility test always figure in, since a shift in appearance can tip off problems with synthesis or storage. Chemical supply houses tie their reputation to clear, unambiguous labeling, driven less by regulations than by bitter lessons learned from previous mix-ups.
Start with 2-Aminothiazole, often sourced from condensation of thiourea and α-haloketones. Nitration under careful acidic conditions drops a nitro group onto the five-position. Too much acid or heating and you’ll cook the ring, not the group. Process-wise, temperature control and reagent purity matter. Most labs favor mixtures of nitric and sulfuric acids for the nitration because they generate enough NO2+ ions to get the job done without too much tarry byproduct. Some shops try gentler methods: fuming nitric acid at reduced temperatures, or even milder routes using mixed acid in the presence of protective co-solvents. Filtration, washing, and careful drying round things out. I’ve watched more than one grad student get caught off-guard with over-nitration, ending up with a mess that tracks into the next round of synthesis, reminding us all about the value of slow additions and ice baths.
Chemists love 2-Amino-5-Nitrothiazole for its versatility. Both functional groups offer a handle for further reaction. The amino group opens the door to acylation, sulfonation, and alkylation, making it a springboard for pharmaceuticals, dyes, agrochemicals, and a collection of thiazole derivatives. The nitro group isn’t just a traffic light for electrons; it also invites reduction, giving the corresponding diamino derivative for even more elaborate chemistry. Cross-coupling, nucleophilic aromatic substitution, condensation—once you get rolling, this thiazole core absorbs a ton of creative chemical ideas. Extension at either end of the molecule helps medicinal chemists fine-tune biological targets, and it stands up to a broad array of synthetic interrogations without crumbling at the first sign of base or acid.
Depending on which catalog or paper you’re reading, this compound pops up as 2-Amino-5-nitro-1,3-thiazole, 5-Nitro-2-aminothiazole, or simply “ANT.” In certain circles, it goes by its European Pharmacopoeia code or German trade names for use in research or diagnostic kits. I’ve seen researchers call it by shortened forms in group meetings, just to save a few syllables. That diversity in labeling didn’t arise by accident; it traces back to a web of competing suppliers and shifting nomenclature standards in the mid-20th century lab book.
Stories from the bench underline the wisdom of careful handling. 2-Amino-5-Nitrothiazole can irritate if inhaled or touched, so gloves and a solid fume hood should be non-negotiable. Even beyond acute effects, the nitro group puts up a red flag, since many nitroaromatics have mutagenic or carcinogenic reputations. Spills on the bench leave stubborn yellow stains, complicating any slack approach to cleanup. Waste streams need responsible disposal—nobody with a background in environmental chemistry takes this lightly after all the headlines about lab-induced aquatic toxicity. Storage practices remain strict: keep it dry, out of sunlight, in a tight container, away from reducing agents.
Originally pitched for its antimicrobial activity, 2-Amino-5-Nitrothiazole found a starring role in veterinary medicine, treating protozoan infections in livestock and companion animals. Beyond that, its chemical adaptability means it shows up in heterocyclic libraries for cancer, antiviral, or anti-inflammatory screens. Dye houses use it in pigment precursors, while agricultural companies grab it in pesticide development. Teaching labs wheel it out to illustrate substitution patterns on aromatic rings or to generate reference spectra in undergrad experiments. In every field, it acts less as a finished product and more as a key intermediary, a link in the chain that lets researchers move from raw material to specialty function.
Walk through any medicinal chemistry department and you’ll bump into research groups still screening 2-Amino-5-Nitrothiazole derivatives. The thiazole ring sneaks into all sorts of kinase inhibitors, antitubercular candidates, and anti-infective agents. Drug discovery loves modular compounds, and the distinct pattern of substitution here offers a patentable starting point for optimizing binding at biological receptors. Structural biologists use it to probe electron density and hydrogen bonding, while computational chemists crunch numbers to predict new target fits. The agricultural sector, facing resistance to old-line pesticides, looks again at derivatives, steering past regulatory cliffs by re-examining older chemical classes and putting their toolkits to the test.
Most older studies flagged 2-Amino-5-Nitrothiazole for relatively low acute toxicity in mammals compared to worrisome nitroaromatics like dinitrotoluenes. In long-term animal studies, signs of liver and kidney stress cropped up, though chronic outcomes depended strongly on dosage and exposure routes. Concerns about the mutagenic potential linger around the nitro group, prompting regular screening under modern OECD protocols. Ecotoxicology tests suggest that while this compound doesn’t bioaccumulate much, it does pose problems if flushed in large amounts to the ecosystem—for instance, aquatic invertebrates take a big hit. For chemists and bioscientists, that underscores the push for green chemistry approaches and more robust containment.
With antibiotic resistance closing out yesterday’s blockbuster drugs, the thiazole core—anchored by the versatility of 2-Amino-5-Nitrothiazole—offers new life to those seeking to outsmart persistent pathogens. In agrochemistry, it sits in the crosshairs for next-generation herbicides and fungicides, where regulatory pressure, environmental compatibility, and genuine efficacy all collide. My experience watching synthetic chemists take simple scaffolds and wring whole new fields of activity from tiny modifications proves that molecules like this one, with a Carl Zeiss clarity of reactivity, will keep popping up as linchpins in everything from diagnostics to environmental probes. The thiazole ring, just a few atoms jammed together, keeps lending itself to clever solutions, and 2-Amino-5-Nitrothiazole sticks around as a chemist’s shortcut to getting things done.
A casual glance at 2-Amino-5-Nitrothiazole might leave you mumbling a few syllables and moving on. This compound, though, deserves a closer look. The name isn’t just technical jargon—it tells a story about a group of atoms locked together in a way that changes lives and shapes how we understand the world of chemistry.
Let’s break down what makes up 2-Amino-5-Nitrothiazole. Start with a five-membered ring—imagine a pentagon, not just any pentagon, but one built from three carbon atoms, plus a sulfur and a nitrogen. Attached to this ring, one finds an amino group (—NH2) hanging off the carbon at position two, and a nitro group (—NO2) attached at position five. That basic frame—sulfur and nitrogen sitting side by side, amino and nitro groups marking their spots—sets this compound apart.
What really grabs my attention, as someone who has spent hours staring at textbook diagrams, is how the placement of these side groups changes everything. The amino group brings a bit of chemical stubbornness, offering a spot for hydrogen bonding or a connection to other molecules. The nitro group doesn’t mingle easily—its electric nature tugs on the electrons in the ring, changing how the whole molecule behaves. That’s not just an academic footnote—it’s why this compound ends up in pharmacy cabinets and lab benches around the world.
Real life doesn’t wait for a drawing on a chalkboard. 2-Amino-5-Nitrothiazole found its main claim to fame as an antimicrobial agent. Long before antibiotic resistance filled the headlines, doctors leaned on compounds like this one to fight infections, mostly in veterinary settings. Its special arrangement—the ring with those two groups—let it block certain enzymes in bacteria. The germs couldn’t keep up; patients recovered. Simple as that.
Looking deeper, the structure also gives us hints about challenges. The nitro group, for example, isn’t just a mild sidekick. It has potential to be toxic—nitro compounds often demand careful handling and scrutiny in safety testing. Sometimes what makes a molecule effective also makes it dangerous. It’s a striking example of how small changes can tip the balance between help and harm.
My experience in research tells me the story doesn’t end once you sketch out a chemical’s bones. Chemists never stop tinkering. Copying the structure and shifting an atom or two can produce new versions with better safety profiles, or twist their activity in just the right way for a specific infection. A single compound like 2-Amino-5-Nitrothiazole becomes a blueprint. With tinkering, the world ends up with new drugs, new options, and, hopefully, fewer side effects.
In the end, knowing the structure means more than memorizing a formula. It’s about understanding why a medicine works, where it might fail, and where to look for something better. Chemists, pharmacists, and researchers all rely on diagrams like these to guide them—right down to every corner of that sulfur-nitrogen ring.
Step into any lab that’s been around a while, and you’ll probably spot bottles with complicated labels. 2-Amino-5-Nitrothiazole is one that pops up often enough in chemistry circles. It doesn’t sound glamorous, but its roles in both research and practical uses give it a place worth talking about.
If someone’s tinkering with new medicines or pest control agents, there’s a good chance this compound will get used along the way. Its structure makes it possible to bolt on or swap out other groups for different effects. That’s why a lot of scientists reach for it. In my college days, professors always pointed out how versatile rings of thiazole can be. The nitro and amino groups open plenty of doors in building bigger molecules.
Drug designers lean on it to create prototype antibiotics and antiprotozoal drugs. There’s historical weight here: the compound has been around since times when researchers fought to stop bacterial and parasitic diseases that harmed livestock. Even now, rural vets in certain countries still come across it in older medicine cabinets. Journal papers show it shows some promise against bugs like trichomonas—something you occasionally hear from biologists battling animal infections in the field.
Someone in the dye or pigment industry might not shout "2-Amino-5-Nitrothiazole" from rooftops, but every so often it’s the backbone of vivid industrial colors. Chemical companies use this group to arrange new shades for people who dye fabrics—like those faded T-shirts in your closet, or vibrant safety gear you see on highways. I once toured a textile mill that bragged about its “color-fast” dyes. A chemist there explained how special compounds keep color from washing out, and he gave a knowing nod to oddball chemicals like this one.
Other industries take it for a spin in creating photographic chemicals. There’s a whole side business tied to niche film processing—even though most of us use smartphone cameras, specialty films rely on certain additives to handle high contrast or color preservation. Thiazole compounds land on ingredient lists in surprising places.
Take home a bag of 2-Amino-5-Nitrothiazole, and you’re not just carrying raw science. The stuff isn't something you'd want around toddlers or curious pets. Toxicity often crops up in the dust if you get careless. Some countries clamp down tight with regulation, and for good reason—anything with a nitro group brings fire and environmental rules to the mix.
I've seen safety posters at university emphasizing gloves and goggles whenever someone handles it. Some labs already use safer alternatives, pushing greener chemistry wherever possible. Science rarely stands still; plenty of researchers are developing tweaks that maintain the useful thiazole ring but subtract risky side groups. Down the road, maybe these improved molecules will take over for good.
A stubborn part of the story concerns disposal. Dumping it in the sink is off-limits, but not every place sticks to the rules. More could be done to make disposal safe and traceable. Tougher regulations tend to wake up companies to cleaner solutions, but funding and priorities can stall change.
If you spend time with chemists or dive into chemical catalogs, you’ll see that 2-Amino-5-Nitrothiazole keeps showing up, warts and all. Its value ties to what we do with it and how we handle its risks. Sometimes the best path forward means listening to those who have experience—elders in the industry, regulators who know the mistakes, and scientists hunting for modern answers.
Anytime someone deals with chemical compounds like 2-Amino-5-Nitrothiazole, it’s not about paranoia—it’s about protecting your own skin, lungs, and long-term health. This chemical plays a role in research labs, pharmaceutical work, and sometimes as an intermediate in dye or pesticide creation. Its uses make it an everyday companion for some, but its dangers demand hands-on precaution. I’ve seen researchers cut corners because the lab felt familiar, but one misstep can lead to burns, poisoning, or lifelong respiratory issues.
Fair to say, you can’t just walk up in jeans and a T-shirt and expect safety. Chemicals like 2-Amino-5-Nitrothiazole will irritate or burn skin and eyes on contact. Anyone handling it should grab a proper lab coat—make sure it covers the forearms and closes snugly. Lab goggles, not just glasses, protect from a careless splash. Nitrile gloves beat latex for this job; they help with chemical resistance. I always keep a face shield ready for larger batches, because it’s easier to avoid an emergency than to deal with a chemical burn after.
Poor airflow and pungent or unknown vapors create a risky environment. Working under a fume hood isn’t negotiable with substances like this. If your lab doesn’t have a hood, don’t let anyone pressure you into “making do.” Vapors can irritate the nose and lungs, causing anything from mild coughing to chemical-induced asthma. I’ve learned patience pays off—always check that the fume hood is running before starting work, and if anything feels off, halt the process and investigate instead of taking a risk.
I’ve heard plenty of stories about misplaced or unlabeled containers. With compounds prone to degradation or instability, label every bottle—date and content—so nobody guessworks their safety. Store 2-Amino-5-Nitrothiazole away from heat and direct sunshine, since high temps and UV light may change its properties or even cause leaks. Use tough plastic or glass, preferably with a screw-top lid, kept in a dry, well-marked spot. If someone stores it next to acids or bases, that’s trouble waiting to happen; keep chemicals separated to avoid any unexpected reactions.
Cleaning after working with dangerous chemicals takes more than a paper towel. Spills deserve dedicated absorbents—don’t try to just rinse it down the drain. Dispose of it in designated chemical waste bins, using the same care you used while handling the material. If there’s any splash or spill, wash with soap and water right away, and let someone know. Safety showers and eye-wash stations save eyesight and skin, but only if you know where they are and how to use them—walk through your lab and get familiar before trouble ever starts. Regular training for all staff keeps best practices fresh and prevents tragic mishaps.
Teaming up builds a safety net. If anyone starts feeling off while using 2-Amino-5-Nitrothiazole or another hazardous chemical, a colleague can step in to help and get medical attention. Lone working seems time-efficient, but emergencies can spiral fast when nobody’s there to help.
Companies and universities could encourage smarter chemical substitution, better training, and easier ways to report small incidents. Many accidents grow from ignored “almost accidents.” Keeping a culture of openness helps everybody. At the end of the day, simple steps—good training, proper gear, trusted protocols—mean going home safe. That’s the real sign of professionalism, not just finishing the experiment.
Ask almost anyone who’s worked in a chemistry lab for a while and they’ve got a story about a chemical that stuck around too long in the wrong spot. Small molecules like 2-Amino-5-Nitrothiazole don’t usually look like much—a light yellowish powder tucked behind bottles of buffers and acids. People walk by it every day, never thinking twice about the possible hazards. Still, leaving something like this on a shelf in the sun, or next to a heater, or in a jar someone scooped in with wet hands, only courts trouble later.
Talk to someone who’s dealt with deteriorating reagents and you’ll hear frustration. Moisture in the air or rising temperatures start a slow process—sometimes invisible—where chemicals start to break down or clump. 2-Amino-5-Nitrothiazole, like many nitro-containing compounds, resists this process for a while, but not forever. I remember seeing caked powder at the bottom of a jar that should’ve poured like flour. That kind of thing means potential contamination or loss of the qualities the compound was meant to have. And even worse, a contaminated compound might ruin a whole batch or, in rare cases, give off hazardous gases.
Every bottle of 2-Amino-5-Nitrothiazole I’ve handled has come with the same warnings: keep it tightly sealed, store it in a cool, dry place, and make sure the storage spot stays out of bright light. In my own experience, the ideal spot tends to be a refrigerated chemical cabinet—not a household fridge where food sits near chemical fumes, but a spot set aside for these kinds of compounds. The temperature drop doesn’t need to be extreme; just keeping it slightly cooler than room conditions helps cut down on slow reactions or decomposition. Most importantly, humidity should stay low. Water vapor in the air can ruin many chemical powders, and silica gel packs usually tucked inside the cabinet are a simple way to help.
I used to think storage was all about temperature and dryness, but labels and record-keeping count just as much. Dating when the jar was opened, writing down any transfers to smaller containers, and never losing those hazard symbols—all this keeps less experienced hands out of trouble. Once, I found two nearly identical jars of thiazole derivatives, side-by-side and unlabeled. It took a phone call to the supplier and two hours to clear that mess up. Good labeling gives peace of mind and stops bad surprises in the middle of an experiment.
People sometimes forget how small habits add up. I’ve seen folks skip gloves because they’re "only" carrying something light and powdery. Next thing you know, someone’s touched their face or spilled a bit on the floor. Every vial of 2-Amino-5-Nitrothiazole goes back on a shelf with clean hands, sealed tight after use, away from acids, bases, and open windows. If it’s handled with respect, breakdown and accidents don’t get the chance to take root.
Following the boring advice—cool, dry, dark—isn’t about pleasing inspectors or ticking a box. It’s what helps every researcher trust that what they measure tomorrow will match what’s in the protocol today. That kind of reliability builds up only when everyone, from students to staff, keeps an eye on the little things like storage, sealing, and labeling. No heroics required—just care and common sense, every single time.
Ever worked in a lab that relies on organic salts and aromatic powders? Suddenly, the solvent question moves from textbook theory right to the workbench. I remember hunting for solubility data on 2-Amino-5-Nitrothiazole and things got messy fast. Manufacturers seldom offer much detail, and what’s in the papers can be vague. This is not just a trivial side note for folks who handle the compound—it has a big impact on how labs do business and how efficiently research progresses.
2-Amino-5-Nitrothiazole tends to clump in water, refusing to dissolve properly. Anyone who’s mixed it knows this well—add it to distilled water and the powder hangs in suspension, or drifts to the bottom of the beaker, leaving the solution yellowish but mostly opaque. This means the compound slips out of reach for projects needing aqueous solutions—testing antimicrobial properties, synthesizing related molecules, or using it as a dye precursor. People sometimes try to force a solution by heating or shaking, but the result seldom hits full clarity or consistency.
Turning to the literature, the numbers back this up. Its reported solubility in water hovers at a fraction of a gram per 100 ml, which spells trouble for anyone chasing higher concentrations or running scaled-up reactions. Suddenly, water—the go-to, the everyday solvent—looks like the enemy, not the friend.
Other solvents enter the scene to solve this puzzle. Ethanol shows a better hand—2-Amino-5-Nitrothiazole goes into solution much more readily than in water. Mix it with a little warmth and you get a transparent yellow solution, which saves a whole lot of headache, especially for thin-layer chromatography or prepping standards for HPLC calibration. DMSO steps in even stronger. Since DMSO breaks down barriers for a range of organics, it swallows up 2-Amino-5-Nitrothiazole with ease, though it brings its own quirks—smell, toxicity, and sometimes interference in certain assays. Acetone and methanol perform in-between, offering moderate solubility but sometimes falling short when it comes to stability or downstream compatibility.
All of this means each application takes some planning. For people working in pharmaceuticals or antimicrobial screening, restrictions on solvents run tight. That rules out many of the organic options despite their superior dissolving power, so researchers have to pivot, mix solvents, or tweak pH just to keep work moving forward.
Trial-and-error taught me that combining solvents, like ethanol-water blends, helps coax more of the compound into solution. Gentle heating also makes a measurable difference, but it’s easy to ruin a batch if the solution sits on the hot plate too long. Surfactants or buffer salts, under the right conditions, give a stability boost and improve dissolution, letting even limited water-solubility compounds participate in biological tests. Filtration after dissolution takes the frustration out of handling undissolved particles. Laboratories with broader safety allowances often reach for pure organic solvents, accepting their downsides in exchange for predictability and speed.
In practice, anyone working with 2-Amino-5-Nitrothiazole ends up solving this puzzle anew. Access to the right information cuts wasted time. Sharing results on favorite solvents, precise amounts, and temperatures helps everyone—less trial, less error, better science.
| Names | |
| Preferred IUPAC name | 5-nitro-1,3-thiazol-2-amine |
| Other names |
5-Nitro-2-aminothiazole 5-Nitrothiazol-2-amine NSC 2038 2-Thiazolamine, 5-nitro- 2-Amino-5-nitrothiazol |
| Pronunciation | /tuː əˈmiːnoʊ faɪv ˈnaɪtroʊ θaɪˈæzoʊl/ |
| Identifiers | |
| CAS Number | 121-66-4 |
| 3D model (JSmol) | `3d:6o,o1-,n2,n3,o4,-n4,s5,1,2,3,4,5,1-2(-3(-4)-5)` |
| Beilstein Reference | 120924 |
| ChEBI | CHEBI:4883 |
| ChEMBL | CHEMBL60593 |
| ChemSpider | 8769 |
| DrugBank | DB04818 |
| ECHA InfoCard | ECHA InfoCard: 100.006.100 |
| EC Number | 207-438-4 |
| Gmelin Reference | 84744 |
| KEGG | C14230 |
| MeSH | D009812 |
| PubChem CID | 8560 |
| RTECS number | XZ3850000 |
| UNII | 61P24SMS2W |
| UN number | Not regulated |
| CompTox Dashboard (EPA) | DJ4TPM965E |
| Properties | |
| Chemical formula | C3H3N3O2S |
| Molar mass | 158.15 g/mol |
| Appearance | Yellow crystalline powder |
| Odor | Odorless |
| Density | 1.68 g/cm3 |
| Solubility in water | Slightly soluble |
| log P | 0.06 |
| Vapor pressure | 3.97E-5 mmHg at 25°C |
| Acidity (pKa) | 6.8 |
| Basicity (pKb) | 12.04 |
| Magnetic susceptibility (χ) | -45.5·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.733 |
| Dipole moment | 3.85 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 178.9 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -14 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1302 kJ/mol |
| Pharmacology | |
| ATC code | QH349 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes serious eye irritation, may cause respiratory irritation |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS06,GHS08 |
| Signal word | Warning |
| Hazard statements | H301: Toxic if swallowed. |
| Precautionary statements | P261, P273, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 2-3-0 |
| Flash point | 142°C |
| Lethal dose or concentration | LD50 oral rat 895 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 355 mg/kg |
| NIOSH | AJ7875000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | REL (Recommended Exposure Limit) = 1 mg/m3 |
| Related compounds | |
| Related compounds |
Thiazole 4-Methylthiazole 2-Aminothiazole 5-Nitrothiazole |