Back in the early days of heterocyclic chemistry, researchers dug through thiazoles to find compounds that could make a real difference in medicine, agriculture, and industrial processes. In the 20th century, 2-Amino-5-Methylthiazole (CAS 7305-71-7) stood out—not via an accident, but after years of fine-tuning and searching for molecules that could be tweaked to serve multiple uses without triggering unnecessary headaches for workers and researchers. I’ve walked plenty of labs where researchers—some with dozens of years under their belts—pointed to thiazole derivatives as stepping stones toward blockbuster drugs. Over time, 2-Amino-5-Methylthiazole attracted pharmaceutical chemists, not for its flashiness, but for its potential as a core building block. Long before anyone worried about social trends or buzzwords, chemists needed precursors that worked and didn't break the budget. This compound landed in research notes for just those reasons.
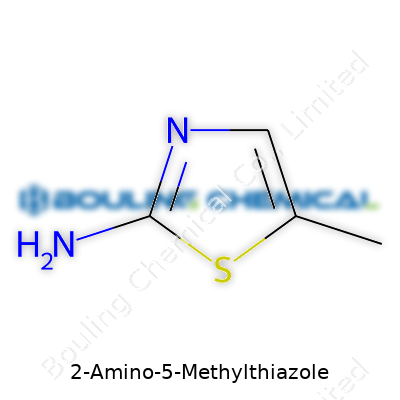
In a world full of complicated specialty chemicals, 2-Amino-5-Methylthiazole isn’t exactly a rockstar, but it’s a trusty workhorse. With its anchor-like five-membered ring, an amino group juts out at position two, and a methyl group clings to carbon five. This structure gives it a personality—small, but packed with possibilities. The solid form packs easily, but don’t expect huge batches lying around; instead, you’ll often find it bottled up, ready for precise use. Over the years, I’ve seen small-scale custom syntheses win out over generic, shelf-stable alternatives because this molecule doesn’t hog space or require fussy handling.
2-Amino-5-Methylthiazole hangs at room temperature as a beige or pale yellow powder. It dissolves easily in hot water and some organic solvents, a convenience that helps during experimental protocols. With a melting point in the 110-115°C range, it easily melts during many standard lab operations but won’t vaporize or decompose right out of the vial. The smell—pungent and acidic—reminds you it belongs to the thiazole family. Anyone who’s weighed it on a balance with drafty lab doors knows that stable handling wins out, since it doesn’t run off into the atmosphere. Its moderate solubility and well-behaved reactivity turn it into a staple for incremental progress in the lab.
Most suppliers offer 2-Amino-5-Methylthiazole at assay purity levels exceeding 98%. Any reputable label should spell out batch number, manufacturing date, and storage advice. In labs I’ve worked in, trusted procurement officers look for safety data sheets that don’t fudge the numbers—every bottle should detail hazards, handling tips, and breakdown products. Sometimes, unscrupulous suppliers try to cut corners, leading to grief down the road. That’s why so many colleagues double-check lot analysis. Labels clearly state molecular weight (114.16 g/mol), with chemical formula C4H6N2S, and supply standardized hazard warnings in line with GHS protocols.
Most commercial synthesis routes build on the condensation of acetamidine hydrochloride with methyl bromopyruvate or methylthiourea. Older methods used more waste-heavy options—something anyone trying to keep lab waste manageable will appreciate transitioning away from. Over years of bench work, chemists have improved reaction yields and streamlined purifications, usually by kicking in a catalyst or precise pH adjustment. From small-pilot syntheses to scaled-up batches, repeatable protocols matter for both cost and peace of mind.
This molecule brings a toolkit for organic chemists. The amino group gives the possibility of acylation and sulfonation, while the thiazole ring can take halogenation or forms the basis for Suzuki-type couplings. Over dozens of project cycles, modifying this scaffold let colleagues access powerful intermediates for everything from kinase inhibitors to dye precursors. In medicine discovery, scientists commonly swap out substituents or extend the ring to test new activities. During fail-and-learn cycles, this base offers a forgiving foundation: it doesn’t easily decompose or overreact unless deliberately forced.
You’ll spot 2-Amino-5-Methylthiazole under several aliases: 5-Methyl-2-Thiazolamine, 5-Methyl-2-Aminothiazole, AMT, and Thiazol-2-Amine, 5-methyl. Commercial catalogs don’t settle on just one, especially when imported from different regions. For anyone in procurement or regulatory tracking, keeping an eye on alternate names saves time—but it also prevents disastrous double ordering. Product variety helps with global supply chains, though researchers sometimes vent about confusion caused by untidy naming conventions.
Handling this compound demands respect. Although not as toxic as notorious industrial chemicals, 2-Amino-5-Methylthiazole does cause irritation if inhaled or contacted directly. I once watched a new graduate misjudge air currents and brush up against a pile of unswept powder—skin reddened, nothing major, but a silent reminder. Material safety data recommends gloves, goggles, and routine fume hood use during weigh-outs. Standard practice keeps quantities small, bottles sealed, and bench tops clean. Most experienced workers keep a spill kit nearby and insist on prompt cleanup.
Its main claim to fame is straightforward. Drug designers pick it as a precursor for antifungal, antibacterial, and anti-inflammatory agents. Agricultural chemists treat it as a sidekick in the race to beat crop pathogens. Some dye manufacturers tap it for new pigment families, especially those needing fast synthesis without elaborate intermediate steps. Industrial research projects often pull it from the shelves for custom syntheses of new thiazole derivatives. Anyone focusing on early-stage structure-activity studies will recognize its familiar outline in literature, referenced again and again across disparate journals.
In R&D, scientists search for new ways to use this backbone as a launching pad. In my own teams, a fair amount of the brainstorming process starts by swapping groups off the amino or methyl site, testing each tweak in an assay or controlled setting. Wide-ranging research clubs push boundaries, checking old patents and looking for unexplored applications—perhaps extending into the field of sensors, or as ligands in coordination chemistry. In startup labs trying to outpace deadlines, researchers write grant applications citing 2-Amino-5-Methylthiazole precisely because it supports rapid prototyping and chemical diversification.
Toxicologists still watch it closely; short-term exposure won’t knock most users off their feet, though high doses and chronic handling bring up concerns. In animal models, moderate oral toxicity appears, and metabolic breakdown doesn’t linger much, but careful record keeping matters. Regulatory guidelines say keep exposures low, especially for anyone with respiratory sensitivities or dermatological conditions. Studies in cell cultures flag possible DNA or protein-interaction risks at high concentrations—enough to warrant caution, particularly in labs with a high turnover of early-career experimenters. Safety committees always urge erring on the side of less, not more.
With growing demand for new antimicrobials and crop protection agents, interest in thiazole derivatives is only climbing. As more synthetic biology projects hunt for unique chemical frameworks, 2-Amino-5-Methylthiazole stands ready for action. AI-driven drug discovery platforms pull reference compounds from thiazole libraries, betting on past performance and attainable chemical diversity. From my vantage point, chemical companies and university labs alike see room for new analogues built off this foundation, especially as green chemistry methods improve overall sustainability. The path forward isn’t without bumps—better labeling, safer large-scale handling, and expanded research on low-level toxicity matter a lot. Yet the core appeal remains: it’s a reliable workhorse with enough flexibility to keep pace with ambitious new science.
Chemistry classrooms usually throw around chemical formulas as if everyone can see the molecule in their mind. For 2-Amino-5-Methylthiazole, it’s a mouthful until you see what makes it up. This compound carries the formula C4H6N2S. Each letter and number here marks the count for carbon (C), hydrogen (H), nitrogen (N), and sulfur (S) that stick together in this thiazole-based molecule.
It sometimes surprises folks how much can hinge on just a tweak in a formula. Shift a carbon or swap a hydrogen on the structure and suddenly you’ve got a different substance entirely—a fact hammered home to me in university organic labs where the difference between success and disaster was a single atom to the left or right.
We’re not talking big numbers here, but small changes pack punch. The molecular weight for 2-Amino-5-Methylthiazole sits at 114.17 g/mol. To put it plainly, this value matters because it decides how you measure the compound, how you dissolve it, and how it travels if you send it through your body or environment.
Anyone running experiments in a lab comes to respect molecular weights quickly. Solutions don’t mix properly if you guess wrong, and reactions go off track. For pharmacy and biotech, even the tiniest miscalculation at this step means ruined batches—years of funding flushed away. Beyond the lab, emergency spill teams rely on this figure when dealing with leaks, since it points to how fast a chemical spreads and how it interacts with cleanup supplies.
This thiazole derivative shows up in projects searching for new antibiotics and other drugs. Its compact formula means it squeezes into places bulkier molecules can’t reach, giving it a competitive edge in medicinal research. Additions like the amino and methyl groups change how it behaves compared to its chemical cousins.
I once shadowed a small pharmaceutical team looking to tweak thiazole rings to fight a stubborn infection. They spent weeks testing side chains, but kept circling back to 2-Amino-5-Methylthiazole’s profile. Its low molecular weight helped it move through membranes. That property made it easier to play around with, speeding up synthesis and testing time. These small wins add up, especially when medicine races against resistant bacteria.
With so many chemicals traded and researched each year, confusion creeps in over names and formulas. Even in journal articles, typos and swapped numbers crop up. Open communication between labs, clear safety data sheets, and double-checking sources cut down on these mix-ups. Technology can help—barcode-tracking and updated digital inventories now catch mistakes before someone grabs the wrong jar. Still, people on the ground need a keen eye for detail and a no-shortcut attitude.
Careful labeling, peer-reviewed fact checking, and regular training smooth out communication snags. Regulatory agencies also play a part; clear rules for reporting chemical names and weights keep standards high across borders. The push for open-access databases with verified data lets smaller labs skip guesswork and work confidently. Having these resources can mean the difference between a successful project and lost time tracking down a tiny molecular detail.
Some chemicals quietly steer the course of medicine and technology, even though most people never hear their names. Take 2-Amino-5-Methylthiazole. Most folks haven’t run into it outside a laboratory. Still, small compounds like this one shape plenty of products we rely on every day.
Years ago, someone handed me a sample bottle of 2-Amino-5-Methylthiazole in a research lab and simply grinned, saying, "Watch where this ends up." Back then, I didn’t expect such a modest molecule to wield real influence. Chemists favor it as a building block for all sorts of medicines. Digging into it a bit, I noticed how it fits like a puzzle piece in antibiotics, anti-inflammatory drugs, and treatments for diabetes. Those thiazole rings help scientists piece together larger, more complicated drug molecules. When a new antibiotic faces resistant bacteria, there’s a chance it owes part of its structure to this thiazole compound.
That’s not just a lab story, either. Pharmaceutical companies look for reliable, cost-effective starter materials. 2-Amino-5-Methylthiazole doesn't break the bank, the chemistry is straightforward, and it's pretty adaptable for inventing molecules with new properties. With global health relying so much on new and better treatments, it’s tough to overstate how critical good chemistry tools are.
Academic labs and industrial teams both lean on this compound. If the push is to make dyes, corrosion inhibitors, or even advanced materials that light up under UV, the thiazole’s backbone keeps popping up in published recipes. On the research side, what stands out is flexibility. The compound acts kind of like a Swiss Army knife: want to build something that resists breakdown in harsh conditions, or lights up for imaging tests? The basic structure gives scientists a head start.
Farmers have skin in the game here, too. 2-Amino-5-Methylthiazole underpins several compounds in agrochemical products that treat or guard against plant diseases. Crops battle fungus and bacteria year in and year out. The molecules that fight those blights often get pieced together from thiazole building blocks. If a new fungicide reaches fields and helps preserve a wheat or rice harvest, chemists may have turned to this compound in the design phase. Years of harvests depend on such behind-the-scenes helpers.
No story about a widely-used chemical is complete without confronting the challenges tied to safety and production. In my brief spell working in an industrial setting, conversations circled back to waste handling and risks. Questions pop up: Are there greener ways to make it? Can the process avoid toxic leftovers? Engineers and chemists both recognize the need for sustainable, careful reactions. Companies are circling back to older recipes, searching for cleaner routes using less solvent, and looking at recycling methods for by-products. Each little improvement makes a difference. As more countries clamp down on industrial waste, chemical plants face real pressure to adjust these syntheses.
2-Amino-5-Methylthiazole sounds like the kind of compound only a scientist would care about. Yet, each bottle fuels research advances, turns ideas into life-saving drugs, and runs through processes in agriculture and specialty manufacturing. When a chemical keeps popping up in cures, crop protection, and research, it earns a permanent place on the shelf—and in conversations about progress.
2-Amino-5-methylthiazole might not sound familiar to people who don't spend much time in a lab, but for researchers and folks in the pharmaceutical world, it shows up pretty often. I’ve seen it sitting in storage cabinets, waiting its turn for use in making drugs and complex molecules. Even if you’re only dealing with small samples, treating this chemical right keeps both people and experiments safe. It’s easy to forget about safety when the tasks become routine, but small mistakes in storage can ruin projects and even cause real harm.
In my experience, storing chemicals like 2-Amino-5-methylthiazole boils down to a few basic rules. It doesn’t handle moisture well. Let it absorb water from the air, and you might see clumping or chemical breakdown, which wrecks any hope of consistent results. Once, a lab I worked with had a stash of this compound get contaminated because someone left the cap off just for an afternoon. The next bottle came with an airtight lid, and people took the hazard seriously after that.
The best way to store this compound comes down to a cool, dry place. If you can keep it below 25°C, even better. Most labs lean on a reliable refrigerator or a well-controlled storage room. Some instructions suggest using a desiccator if the air feels humid—silica gel packets tucked around the bottles help pull any stray moisture away before it settles in the powder.
Ignoring proper storage might seem easier when things get busy. I’ve seen folks stack chemicals out in the open, too close to the sink, convinced nothing bad will happen. Sooner or later, a spill occurs, or worse, the quality drops. With 2-Amino-5-methylthiazole, compromised product doesn’t just waste money; it can force researchers back to square one with weeks of lost work. Nobody wants to redo an entire synthesis because a bottle went bad under the wrong conditions.
Some might roll their eyes at handling guidelines, but it’s all there for a reason. Maybe the best example comes from an old colleague who learned to double-check every label and cap after an expensive accident in his grad school days. In the end, the best storage plan is simple: airtight containers, cool shelves, low humidity. Labels need to show the date and contents with no room for guesswork. I even got in the habit of giving every bottle a once-over before use—no point in working with material that’s gone off.
Labs could make it easier to do the right thing by building good storage into the workspace. Shelves above head height don’t help anyone; clear labels and easy access make a difference. Training new team members can keep mistakes from happening. At my last job, a formal checklist on the wall by the chemical cabinet caught oversights before they snowballed into bigger problems.
Storing 2-Amino-5-methylthiazole right makes life easier for everyone—no drama, just work that moves forward.Plenty of folks rarely give much thought to the chemicals floating through labs and factories, but 2-Amino-5-Methylthiazole doesn’t deserve to cruise under the radar. This solid often shows up in pharmaceutical research, dye production, and the hunt for new materials. Pick up a bag of it and it looks unassuming—off-white, maybe just a powder to the untrained eye—but beneath that plain appearance hides a variety of health issues if you don’t show some respect.
Get this stuff on your hands, it starts with just a little irritation or itching. Catch the fumes, and the trouble often starts in the lungs. Anyone who’s spent much time in a poorly ventilated lab knows the headache that creeps in after a few hours with these thiazoles around. If the exposure keeps going, the cough and chest tightness might join in. The science backs it up: occupational studies flag the compound as a possible irritant, and while it hasn’t landed on every major hazardous substance list, caution has become standard in places handling it regularly.
Old-timers in chemistry circles never just scoop out this compound bare-handed. Nitrile gloves are the norm, and folks keep goggles on tight—skin contact leads to rashes faster than you’d think. Don’t forget the lab coat. Wash your hands, even if you only passed by an open container. Accidents happen in seconds, and the residue travels fast.
Workspaces dealing with 2-Amino-5-Methylthiazole run strong ventilation day and night. It’s too easy to ignore, but letting fumes sneak up on you leads to long breaks with pounding heads and tight chests. Fume hoods aren’t just for show—they’re the workhorses saving people day after day.
Spilled powder clings to surfaces and gear. Wipe downs with damp cloths and disposal in the right bins cut down on stray dust. No one wants to sweep blind in a space full of airborne hazards. Large spills call for just shutting the room and letting trained folks in with respirators to sweep it up.
Plain guidelines do plenty, but the real game changer happens when folks watch out for each other. I’ve watched seasoned techs step in before a rookie opens a jar without gloves. That kind of teamwork keeps the stories of chemical burns and hospital trips as ghost tales passed down, not lived out again.
Facilities with proper signage, regular safety drills, and fresh training sessions have a lighter incident log. Factories and research labs already juggling dozens of compounds have learned to treat anything with a thiazole ring as worth a double-check. You want to identify risks before finding them out the hard way.
Chemical safety means more than labels and checklists. It comes down to action, attitude, and the right tools in hand. For 2-Amino-5-Methylthiazole, it makes sense to keep the supply in tight storage with clear hazard information—no unmarked bottles stuffed in the back. Clean workspaces, good airflow, and personal protective equipment keep risk low and workdays uneventful.
The right response to future reports or fresh science on long-term effects is to up the training—not toss warnings in a binder and forget them. Sharing knowledge about what works in controlling exposure, both online and at the bench, holds more value than any dusty rulebook.
Most folks in chemical labs, whether in research or manufacturing, will bump into 2-Amino-5-Methylthiazole at some point. This compound pops up in drug discovery, dye synthesis, and as a building block for other specialized chemicals. On the bench, small differences in quality can throw off experiments or lead to headaches in scaling up production. I've learned to check the small print on the raw materials, as surprises in purity can cause big setbacks down the line.
Stories circulate among chemists about tiny contaminants ruining days of work. Most batches of 2-Amino-5-Methylthiazole offered from reputable suppliers usually promise upwards of 98% purity. Let's not kid ourselves—sometimes that 2% of 'impurities' can pack a punch in a reaction flask, especially if you're chasing trace-level reactions or trying to publish clean spectra. High purity, say 99% or better, generally costs more but brings peace of mind.
Why care about that last percent? Just last winter, I saw a colleague wrestling with an unknown peak in an NMR spectrum, wasting hours before tracing it to a cheap supply of 2-Amino-5-Methylthiazole. Pure starting material keeps those kinds of mysteries at bay, and nobody likes running column chromatography just because the supplier cut corners.
Most of the time, a fresh bottle comes as a pale yellow to light brown powder or solid. Sometimes, you’ll see variations in shade or graininess from batch to batch, which can make you suspicious the first time you spot it. But that color shift usually stems from trace oxidized products or unremoved solvents—rarely enough to throw off the utility for most research. If the stuff rolls out of the bottle as a clumpy, dark-brown mess, there’s room for worry. That might mean poor storage, moisture exposure, or rough purification on the supplier’s end.
Once, I got a batch that just looked off—a bit sticky with an odd smell. Against my better judgment, I tried using it in a reaction. The yield tanked. Lesson learned: if something looks wrong, trust your eyes and don’t compromise on quality. Reliable suppliers mention both purity and appearance on their certificates of analysis for this reason. That piece of paper can save hours of troubleshooting.
Even if most sources claim high purity, it’s wise to ask for an actual COA for your batch, not just a generic one. A direct line to technical support helps if you run into anything strange. Some larger companies have gotten better at offering lot-specific data. That’s been a relief, especially after too many headaches with off-brand batches in the past. For tricky work, an extra purification step like recrystallization can sometimes improve quality at the laboratory level, even if it adds a bit of time to the project.
While price remains a key concern for larger orders, smart labs budget for the better stuff now, having learned from experience. Poor raw material just isn’t worth the hassle—and for 2-Amino-5-Methylthiazole, checking purity and trusting your senses on looks avoids a lot of wasted effort.
| Names | |
| Preferred IUPAC name | 5-Methyl-1,3-thiazol-2-amine |
| Other names |
2-Amino-5-methyl-1,3-thiazole 2-Amino-5-methylthiazol 5-Methyl-2-aminothiazole 5-Methyl-2-thiazolamine 2-Amino-5-methylthiazole |
| Pronunciation | /tuː-əˈmiː.noʊ-faɪv-ˈmɛθ.ɪl-θaɪ.əˌzoʊl/ |
| Identifiers | |
| CAS Number | 7305-71-7 |
| 3D model (JSmol) | `load =C1=CN=C(S1)N/C` |
| Beilstein Reference | 120873 |
| ChEBI | CHEBI:17370 |
| ChEMBL | CHEMBL123105 |
| ChemSpider | 28043 |
| DrugBank | DB08223 |
| ECHA InfoCard | 03f70be4-b5ea-42b0-818f-bc0e55fb9fe2 |
| EC Number | 219-236-2 |
| Gmelin Reference | 78538 |
| KEGG | C06259 |
| MeSH | D015597 |
| PubChem CID | 7279 |
| RTECS number | XZ3150000 |
| UNII | 2U6VZ7KCN7 |
| UN number | Not regulated |
| CompTox Dashboard (EPA) | DJ86N3BP0K |
| Properties | |
| Chemical formula | C4H6N2S |
| Molar mass | 99.14 g/mol |
| Appearance | Light yellow to brown solid |
| Odor | amine-like |
| Density | 1.16 g/cm³ |
| Solubility in water | soluble |
| log P | 0.49 |
| Vapor pressure | 0.0585 mmHg (at 25 °C) |
| Acidity (pKa) | 6.0 |
| Basicity (pKb) | 6.17 |
| Magnetic susceptibility (χ) | -63.0·10^-6 cm³/mol |
| Refractive index (nD) | 1.601 |
| Viscosity | 2.06 mPa·s (20°C) |
| Dipole moment | 3.98 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 166.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -15.2 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3633 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P280, P302+P352, P305+P351+P338, P312, P337+P313, P362+P364 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 95°C |
| Autoignition temperature | 430°C |
| Lethal dose or concentration | LD50 oral rat 860 mg/kg |
| LD50 (median dose) | LD50 (median dose) Oral Rat 2040 mg/kg |
| NIOSH | MW4725000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 mg/m³ |
| Related compounds | |
| Related compounds |
Thiazole 2-Aminothiazole 5-Methylthiazole 4-Methylthiazole 2-Methylthiazole 2-Amino-4-methylthiazole 2-Amino-5-ethylthiazole |