Back in the early twentieth century, the world of organic chemistry thrived on discoveries born from the dye industry’s hunger for new building blocks. One such compound, 2-Amino-5-Chlorobenzothiazole, came into the spotlight thanks to research around sulfur and nitrogen-containing heterocycles. This class of molecules provided a launchpad for pharmaceutical breakthroughs. What started as trial-and-error around benzothiazole cores led to a library of derivatives—2-Amino-5-Chlorobenzothiazole stood out due to its unique balance of chemical reactivity and stability. Chemists recognized it as a key intermediate as research ramped up in aromatic amines during the 1950s and 1960s, pushing the boundaries of synthetic organic methods, especially for antimicrobial and antitumor research.
2-Amino-5-Chlorobenzothiazole shows up as a somewhat pale, off-white solid in most labs. It's not flashy, but it stays consistent from batch to batch—crucial when chemical purity is central to downstream processes. As a functionalized benzothiazole, it’s made up of a fused benzene and thiazole ring—a backbone that features in a host of bioactive molecules. Researchers in agrochemicals, dyes, and pharmaceuticals value this scaffold because it opens doors for further modification. They also appreciate supply chain stability; worldwide manufacturing keeps stocks steady, with suppliers labeling lots meticulously to ensure traceability.
2-Amino-5-Chlorobenzothiazole has a melting point around 210-213°C—evidence of fairly robust intermolecular forces. Solubility trends run low in water but much higher in acetone, DMSO, or DMF, which matches the experience of anyone who has tried to streak out a TLC plate. The molecule stands up to room temperature storage without much fuss, so it won’t degrade or polymerize on the shelf. Aromatic amines like this one often present as faintly earthy solids. That little bit of chlorine tucked onto the ring changes the electron distribution, which makes the molecule a handy candidate for further transformations.
High-purity batches hit over 98% as measured by HPLC, which meets nearly all research-grade expectations. Labels must list molecular mass (184.64 g/mol), CAS number (93-67-4), and structural formula. Proper labeling demonstrates respect for lab safety and regulatory hurdles and matters just as much as the substance inside the bottle. Detailed certificates of analysis back up quality, and batch numbers tie every sample back to manufacturing data, making procurement and troubleshooting transparent. Most packaging uses amber glass vials or sealed pouches to keep the contents dry and stable.
There’s a classic pattern to synthesizing this compound. 2-Aminothiophenol serves as a launch point, reacting with phosphoryl chloride and related agents in the presence of a chlorinating source—for instance, N-chlorosuccinimide. The process usually favors mild temperature control to dictate selectivity. After the main reaction, filtration and recrystallization take care of purification. The method’s popularity owes plenty to its convenience: yields tend to be generous, and the starting materials come cheap, something any bench chemist working on deadline and budget will find comforting.
From an early stage, chemists realized the amino group at the 2-position gave scope for forming amide, imine, and urea derivatives. Nucleophilic substitutions at the chloro position open even more doors and bring in further functional diversity. Metal-catalyzed cross-coupling, say, Suzuki or Buchwald-Hartwig reactions, slot into workflows for making everything from kinase inhibitors to fluorescent tags. Sulfur on the core ring invites oxidation, which leads into studies on electronic properties and reactivity. This kind of versatility means the compound rarely collects dust in storage.
Chemists often encounter 2-Amino-5-Chlorobenzothiazole under different aliases, like 5-Chloro-2-aminobenzothiazole or 5-Chloro-benzothiazol-2-ylamine. Older literature may reference it as NSC 19322 or by catalog codes from well-established chemical suppliers. A familiarity with these variants helps avoid confusion—especially when reviewing patents or regulatory submissions, where small discrepancies in naming can slow down critical research timelines.
Working safely with aromatic amines and halogenated compounds starts with vigilance. Skin and eye contact should be limited; gloves and goggles are not just for show. Inhalation risk remains low at room temperature, but powders should be handled inside a fume hood to avoid risk from dust. Proper waste disposal and spill management become non-negotiable if labs want to pass audits and protect their teams. Material safety data sheets underscore moderate irritation but little in the way of acute systemic toxicity, which takes some pressure off but never invites carelessness.
The pharmaceutical pipeline relies on intermediates like 2-Amino-5-Chlorobenzothiazole to streamline lead optimization. Medicinal chemists prize its structure when aiming for enzyme modulators, kinase inhibitors, and antimicrobial scaffolds. Agrochemical researchers turn to its chlorinated ring for plant protection studies, with a particular eye on fungicide development. Outside of health and agriculture, dye and pigment industries bring this molecule into colorant synthesis. Laboratories working in fluorescence tagging or electrochemical sensor research keep it on hand for modifying surfaces and conjugates. The versatility promised on paper turns up in real-world formulations tested in trials that reach across continents.
Investments in R&D reflect the compound’s wide appeal. Universities and biotech firms look toward new derivatives for oncology and infectious disease therapies, driving forward patent activity and clinical screening. Successful modifications to the core structure often rely on introducing new substituents—electron-withdrawing or donating groups shift bioactivity profiles, making small changes significant. Collaborative projects between academia and industry keep the data pipeline busy, as scientists share findings related to reactivity, safety, and in vivo outcomes. This collective effort helps maintain momentum and invites new entrants to push the chemical’s limits further.
Research addressing its toxicity profile reveals a reassuring picture. Standard assays point to low acute toxicity in mammalian models. Chronic exposure data remains under development, but so far, the molecule does not cause genetic mutations in typical laboratory assays such as the Ames test. Still, with all aromatic amines, a measure of caution stays wise—regulators and lab safety professionals keep a close eye on metabolite formation and long-term exposure. Environmental profiles show the need for responsible waste management, especially due to the persistent nature of halogenated compounds.
Prospects for 2-Amino-5-Chlorobenzothiazole rest on its foundation of chemical flexibility. Medical researchers looking to develop new treatments for diseases—from cancer to antibiotic-resistant infections—find encouragement in every modification that leads to improved selectivity or reduced off-target actions. Industrial efforts to “green” the chemical’s synthesis and handling open the door for more sustainable production. Teams dedicated to smart materials and sensors continue exploring its potential, especially in areas like OLEDs and catalysis, where stable, modifiable heterocycles stand out. The ongoing marriage of academic insight and commercial incentive helps the field look beyond short-term gains and focus on the transformative changes this small but potent molecule can offer.
Spotting the name 2-Amino-5-Chlorobenzothiazole on a chemical inventory paints a picture of a precise tool, not a household item. In everyday language, this compound plays a role behind the scenes in places like pharmaceutical labs and specialty chemical manufacturers. The real action happens early in the creation of new molecules, especially for science chasing breakthroughs in medicine.
Lots of chemists see value in 2-Amino-5-Chlorobenzothiazole because of its role as an intermediate. Through its structure—a benzothiazole ring with amino and chloro groups—a world of reactions opens up. This lets researchers build more complicated compounds. In the field of medicinal chemistry, benzothiazole derivatives have shown effects against bacteria, fungi, and even cancer cells. No surprise, labs working on new antibiotics or targeted cancer drugs keep this ingredient on hand.
For anyone who's ever set foot in a university organic chemistry lab, the painstaking work of assembling larger molecules from small pieces is familiar. Building blocks like this one make that work possible. For example, research details how some benzothiazole derivatives perform as inhibitors for certain enzymes in human disease, which gives doctors new tools to manage conditions that once seemed untreatable. In 2023, a survey in the journal European Journal of Medicinal Chemistry summed up dozens of promising drug candidates based on benzothiazole, including drugs in early testing for neurodegenerative diseases and chronic infections.
Beyond the search for new drugs, this compound has made its way into dyes and materials chemistry. Companies making specialty plastics have explored how benzothiazole derivatives can improve thermal stability or resistance to harsh environments. Firms developing advanced coatings look for qualities that hold up against sunlight, friction, and chemical exposure in manufacturing workplaces—benzothiazoles deliver some of those traits.
Knowledge from the textile and plastics industries suggests that specialty chemicals like this one are crucial for creating high-end products. From polyester fibers designed to last through many washes, to sealants used in cars, adding a few grams of the right compound can make a difference in the final product’s durability.
Work with chemicals like 2-Amino-5-Chlorobenzothiazole carries real responsibilities. Many synthetic chemicals that wind up in rivers or soil can persist over time, sometimes with troubling health effects for both wildlife and people. These concerns have shaped stricter regulations across North America, Europe, and Asia. The European Chemicals Agency, for example, provides guidance and oversight on the production and handling of such substances, emphasizing containment, controlled waste disposal, and exposure limits for workers.
In a university research setting, I watched teams wearing gloves, goggles, and lab coats working with even small quantities of similar compounds. Everyone learns proper chemical hygiene from the beginning. It's no bureaucratic box to check; mistakes—even tiny spills—can put people at risk or pollute water supplies. Responsible companies don’t just meet legal minimums, they invest in training, containment tech, and ongoing monitoring.
Better design means safer, more efficient use. Scientists are investigating alternative routes that use less hazardous reagents or generate less waste. Researchers now talk about “green chemistry,” and that’s not marketing—it's about finding smarter ways to make and use potent compounds without endangering the people or environments involved.
2-Amino-5-Chlorobenzothiazole holds a unique spot in the toolbox of science and industry. As knowledge expands, so does the responsibility to work conscientiously and protect both people and the planet while unlocking new solutions in health and materials science.
Chemicals like 2-Amino-5-Chlorobenzothiazole show up often in research labs and in some manufacturing processes. It can look like a harmless, pale yellow powder, but under the surface, it holds health risks worth real respect. I’ve seen enough people get lazy about lab safety—nobody thinks they’ll have the accident, right up until something stings their skin, or fumes catch them off guard. This compound carries both irritant and toxic properties, and sometimes long-term hazards only show up later.
Dust isn’t always visible. You might not smell a thing, but skin contact and inhalation both cause problems. In my years working around various organic chemicals, I learned to take warnings about respiratory and skin exposure seriously. A few missed precautions can lead to rashes, burning eyes, or far worse. The U.S. National Center for Biotechnology Information notes that many benzothiazoles can trigger skin and mucous membrane irritation, along with possible effects on organs after repeated or prolonged contact.
Good personal protective equipment never goes out of style. That means donning safety goggles, chemical-resistant gloves—nitrile usually does the job—and long sleeves or a lab coat. Once, I saw a colleague skip gloves for “just a quick weighing” and end up with a nasty hand rash. Quick tasks deserve just as much protection as hours-long experiments.
Disposable masks or, in some settings, proper respirators keep dust and powder out of your lungs. Ventilation in the work area becomes more than just a comfort—it’s a line between being safe and rolling the dice with your health. Fume hoods or well-positioned exhaust fans do wonders, especially if a process involves heating or agitation.
Storing chemicals like this in tightly-sealed containers, away from food and drink or heat sources, avoids surprises down the line. Dry, cool corners of well-labeled cabinets reduce the odds of dangerous reactions or spills. Flimsy labeling creates more confusion than you might expect, and guessing what’s in an unmarked jar rarely ends well. Even a quick double-check saves grief.
No matter how careful you are, spills happen. I’ve soaked up my share of chemical puddles—small spills can be wiped up using absorbent pads, wearing gloves, and disposing of them in sealed bags as hazardous waste. Never sweep, blow, or create dust; that only increases risk. Larger spills need chemical spill kits, with proper procedures outlined in clear, available documentation. Labs with solid procedures usually see fewer accidents.
Nobody gets up to speed by osmosis. Clear protocols, thorough training, and easy-to-find safety data sheets give everyone on the team a fighting chance. From handling waste to understanding first aid for accidental contact, hands-on safety drills pay off. Sometimes, asking “what’s the worst that can happen?” keeps people—but especially new hires—on their toes more than any sign on the wall ever could.
People sometimes focus on getting the job done fast. I get it—deadlines push, expectations build. But ignoring safety swaps short-term speed for long-term trouble. Staying on top of precautions with chemicals like 2-Amino-5-Chlorobenzothiazole means looking out not just for yourself, but for everyone sharing your air and space.
The real solution isn’t fancy gear or high-tech fixes. It’s building habits, taking time for checks, never getting too comfortable. That’s the difference between stories with happy endings and ones where things went sideways.
Chemistry lessons rarely get as personal as they do with some of the hard-hitting compounds found in modern research. In the crowded field of heterocyclic molecules, 2-Amino-5-Chlorobenzothiazole often draws attention. It features a fused bicyclic ring system, unveiling possibilities that go far beyond textbooks or theoretical models. Let’s crack open what the chemical structure and formula say, and why they matter in a real-world context.
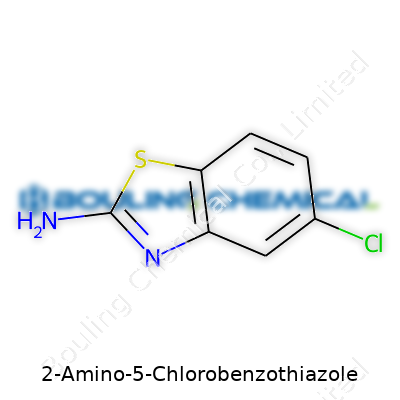
The backbone of 2-Amino-5-Chlorobenzothiazole boils down to a benzothiazole skeleton. Imagine a benzene ring fused directly to a thiazole ring. In this setup, the amino group attaches at the second carbon atom, and the chlorine atom binds at the fifth spot on the benzene ring. The precise structure can be described as 2-amino-5-chloro-1,3-benzothiazole.
Chemically, the structure can be sketched as follows: a six-membered benzene ring, fused to a five-membered thiazole ring holding both sulfur and nitrogen atoms. The amino group (-NH2) sits on the carbon next to nitrogen in the thiazole ring, while chlorine drops onto the fifth carbon on the aromatic ring. This unique pattern creates an electronic environment distinct from the average monocyclic aromatic or thiazole derivatives.
Every undergraduate chemist remembers drawing these rings and counting carbons with a touch of apprehension. For this molecule, it proved useful during a pharmacology stint, where isolating structural analogs with halogen substitutions often led to either boosted or tanked bioactivity.
The formula for 2-Amino-5-Chlorobenzothiazole is C7H5ClN2S. This formula isn’t just a cluster of letters and numbers. It spells out everything a laboratory specialist needs before running thin-layer chromatography or planning nuclear magnetic resonance studies. The presence of both chlorine and sulfur signals extra care in handling, particularly when analyzing elemental composition or combustion byproducts.
Looking at toxicity, these atoms — especially chlorine — often prompt extra gloves and ventilation fans. Over the years, working in academic and applied labs has shown that halogenated benzothiazoles require just as much respect as the latest wonder drug. The formula gives any risk assessment or safety datasheet a reference point.
Why care about such a specific molecule? Research into benzothiazole derivatives, particularly those that incorporate substitutions at the amino or halogen sites, uncovers applications in pharmaceuticals, materials science, and agricultural chemistry. In oncology screenings, for instance, slight tweaks to the structure of compounds like 2-Amino-5-Chlorobenzothiazole sometimes reveal strong anticancer properties.
On the materials side, the electron-rich arrangement this compound sports often leads to unique luminescence or electronic behavior. Colleagues once explored these molecules as organic semiconductors, attempting to bridge gaps that silicon can't reach.
Production calls for strong grasp on synthetic protocols, especially where chlorination and amination steps can trigger side reactions or environmental hazards. Routinely, researchers look for greener methods—sometimes swapping out noxious reagents for milder, lower-waste chemicals. In the future, tighter controls on precursor disposal, better detection of trace contaminants, and employer-supported safety upgrades will keep progress balanced with health and sustainability.
Grasping the core structure and formula opens up more than textbook answers; it signals pathway choices in research and industry, giving users a head start in handling both risks and rewards.
Every chemical on the lab shelf carries a story and a set of risks. 2-Amino-5-Chlorobenzothiazole stands among those substances that look quite innocent in powdered form. It’s used in pharmaceutical research and dye manufacturing, so the odds are good you’ll find it in places with plenty of foot traffic. Anyone who has spent late nights labeling glass bottles for chemistry class or prepping materials in an industrial setting knows the rules around safety often come from lessons learned after someone got hurt.
Inhaling or touching 2-Amino-5-Chlorobenzothiazole is a bad idea. I have seen skin irritation firsthand when someone didn’t notice glove tears. Even small lapses, like leaving a bottle open by accident while chatting with a colleague, can mean days of itchy regret or worse. Chemical safety sheets show risks around the respiratory system and skin, so handling and storage deserve more than a quick glance.
Anyone storing this compound should pick a spot with good ventilation and low humidity. Moisture in storerooms triggers clumping or causes bottles to sweat. I once opened a jar stored beside a leaky air conditioner—inside, the powder caked and clung to the sides, making accurate weighing impossible. Humid conditions also encourage slow breakdown of compounds, so every spill or error adds to long-term risk.
Direct sunlight tends to be a slow destroyer of many chemicals, and 2-Amino-5-Chlorobenzothiazole won’t thank you for a view of the lab window. Take it from my experience: labels fade and material quality shifts. Always put light-sensitive containers in opaque or amber bottles and tuck them on shelves away from windows or harsh ceiling lights.
Flammable solvents and reactive powders should never sit beside this compound. I spent a summer auditing storerooms and lost count of aisles where oxidizers stood a few feet from organics and acids. In the event of a fire, 2-Amino-5-Chlorobenzothiazole breaks down and releases toxic fumes. Store it in solid, sealed containers—preferably glass with tight screw caps. Avoid anything with loose lids or cracked seals.
I’ve seen plastic caps curl and deform after repeated opening and closing: always check the closure before placing a bottle on a shelf. Keep separate cabinets for chemicals with different hazard classes, clearly labeled, and always record each new entry in a logbook. I found that people skip this step, thinking they’ll remember. They never do.
Gloves, goggles, and a clean workspace: these rules never go out of style. Small habits make the difference. I keep a box of disposable gloves on every bench and encourage others to check their hands after handling—one unnoticed spill on a doorknob can spread contamination through an entire building overnight. Dispose of waste in tightly sealed bags, not open bins.
Proper ventilation keeps dust from lingering. Never store 2-Amino-5-Chlorobenzothiazole near food, drinks, or shared spaces. Storage areas with spill kits, absorbent materials, and clear signage let even the most distracted coworker respond quickly if something goes wrong.
Safe storage isn’t a one-and-done job. Review protocols every few months and replace worn-out gear immediately. Include 2-Amino-5-Chlorobenzothiazole in regular audits, and don’t ignore even minor damage to bottles. Making these steps part of the routine means fewer surprises and safer days on the job.
I remember the first time I needed 2-Amino-5-Chlorobenzothiazole for a chemical synthesis project. My mentor grinned, slid over a catalog, and told me to dig into the fine print—how pure, from what supplier, and for what process. Finding out you can’t just order “2-Amino-5-Chlorobenzothiazole” like you do table salt was a wake-up call. The source matters. Purity matters. In one column you have technical grade. In another, research grade. Sometimes pharmaceutical grade pops up, assuming you have deep enough pockets to chase after it.
What does all this mean? The grade you pick tells you how many impurities hitch a ride in your compound. Technical grade tends to cost less, sometimes at the expense of stray byproducts. You’ll spot this batch at dye plants, rubber factories, or industrial labs where a microgram of impurity gets lost among barrels. On the other end, analytical or research reagents undergo extra purification—sometimes by chromatography, sometimes by repeated recrystallization. You won’t want to blow your research funds on technical grade if you’re after clean analytics: for example, prepping an HPLC sample, where a mystery peak can throw everything into confusion.
I’ve had reactions stall thanks to a sneaky contaminant in a bottle of supposedly “high-purity” reagent. In pharmaceutical research, there’s even less room for error. Any impurity can complicate results or worse, interact with target molecules unpredictably. In large-scale manufacturing, trace contaminants might build up when everything scales. That’s why chemists lean so hard on specifications laid out by suppliers. Most reputable manufacturers list purity either as a percentage (like 98%, 99%) or spell out known trace contaminants, often measured by advanced instruments like mass spectrometers or gas chromatographs.
If anyone dreams of regulatory headaches, try submitting a drug application with undefined impurity levels. Quality demands certainty. That principle works its way back through the entire supply chain. Factories running on technical grade might accept a little impurity for speed and savings. In QA/QC labs, a rogue peak could raise alarms, requiring ultra-pure stocks.
The lab notebook never lies. Documenting every bottle’s provenance and stated purity isn’t just paperwork—it's about being able to trace issues if something doesn’t work. In clinical labs, extra cleaning steps or secondary purification add time, but skipping them can put future work or even patient health at risk. I’ve run reactions both ways: unpurified technical grade on a shoestring, pure reagent for exacting experiments. The payoff depends on goals, budget, and regulatory requirements. If the outcome matters, I pick higher purity and inspect the certificate of analysis for each batch.
Thanks to global suppliers, it’s easier than ever to compare purity profiles and batch traceability. I gravitate toward suppliers that publish detailed analysis, not just a purity number. More transparent companies provide methods for purifying their product, and sometimes even feedback from regular customers who point out which grades work best in certain applications.
As someone who’s spent too many hours troubleshooting unexplained results, I root for better standards and more open data on chemicals like 2-Amino-5-Chlorobenzothiazole. After all, the right grade ends up saving money, time, and frustration in the lab.
| Names | |
| Preferred IUPAC name | 2-amino-5-chloro-1,3-benzothiazole |
| Other names |
2-AMINO-5-CHLOROBENZOTHIAZOLE 5-Chloro-2-aminobenzothiazole 2-Amino-5-chlorobenzo[d]thiazole 5-Chlorobenzo[d]thiazol-2-amine |
| Pronunciation | /tuː-əˈmiːnoʊ-faɪv-klɔːr-oʊ-ben-zoʊ-θaɪˈəːzoʊl/ |
| Identifiers | |
| CAS Number | 137-30-4 |
| Beilstein Reference | 172050 |
| ChEBI | CHEBI:35281 |
| ChEMBL | CHEMBL12911 |
| ChemSpider | 16404 |
| DrugBank | DB08309 |
| ECHA InfoCard | 050000017503 |
| EC Number | 212-747-6 |
| Gmelin Reference | 75256 |
| KEGG | C14122 |
| MeSH | D031073 |
| PubChem CID | 69837 |
| RTECS number | DG4375000 |
| UNII | 027A751U00 |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | DF052603 |
| Properties | |
| Chemical formula | C7H5ClN2S |
| Molar mass | 172.64 g/mol |
| Appearance | white to light yellow powder |
| Odor | Odorless |
| Density | 1.48 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 1.91 |
| Vapor pressure | 0.00257 mmHg at 25°C |
| Acidity (pKa) | 6.28 |
| Basicity (pKb) | 6.37 |
| Magnetic susceptibility (χ) | −64.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.708 |
| Dipole moment | 3.56 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 107.1 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -45.34 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3931 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and serious eye irritation, may cause respiratory irritation |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P280, P305+P351+P338, P337+P313 |
| Flash point | 122°C |
| Autoignition temperature | 490°C |
| Lethal dose or concentration | LD50 (oral, rat): 495 mg/kg |
| LD50 (median dose) | LD50 (median dose) of 2-Amino-5-Chlorobenzothiazole is "505 mg/kg (oral, rat) |
| NIOSH | LU9625000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 mg/m³ |
| Related compounds | |
| Related compounds |
Benzothiazole 2-Aminobenzothiazole 2-Chlorobenzothiazole 2-Amino-6-chlorobenzothiazole 5-Chlorobenzothiazole 2-Amino-5-methylbenzothiazole |