The story of 2-amino-4-methylthiazole stretches back over a hundred years, rooted in the early years of heterocyclic chemistry. Synthetic routes for thiazoles started appearing in records alongside the rise of organic chemistry in Europe during the late 19th and early 20th centuries. Its presence in laboratories often came not as a headliner, but as a crucial stepping-stone in exploring sulfur- and nitrogen-containing ring systems. Early chemists discovered that the unique structure—a thiazole ring hosting both an amino and a methyl group—stood out in reactivity, paving the way for more elaborate pharmacological and biological studies. My experience digging through chemical syntheses suggests that as chemical science advanced, 2-amino-4-methylthiazole kept finding new applications in medicinal chemistry, not just as a compound but as a building block.
Pure 2-amino-4-methylthiazole does not attract attention with its appearance—it's often a pale to slightly yellow crystalline powder, subtle in scent. Its molecular formula, C4H6N2S, points squarely to a compact, five-membered thiazole ring. Suppliers usually present it with a purity level above 98%, aimed at researchers or manufacturers involved in pharmaceutical synthesis, agrochemicals, and specialty intermediates. The product typically arrives in tightly sealed bottles, protected from light and moisture, hinting at the care needed to keep its quality intact. Lots are tracked by batch numbers, and reputable sources support their materials with certificates of analysis, spelling out the details of purity, melting range, and other significant traits.
I recall more than one instance when the specific smell of thiazole derivatives gave away their presence in a lab long before measurements. 2-amino-4-methylthiazole carries a melting point of approximately 103–108°C, showing off dependable thermal properties useful in synthesis. It dissolves easily in polar solvents like water, ethanol, and dimethyl sulfoxide, while showing limited solubility in many non-polar organics. Chemically, that thiazole ring supplies a taste for both electrophilic and nucleophilic substitutions. The methyl group brings a bit more electron density, slightly impacting reactivity patterns compared to thiazole rings without such substitution.
Manufacturers typically provide technical sheets highlighting content by weight, moisture content, and trace impurities—a nod to the compound’s popularity among researchers chasing dependable and reproducible results. Labeling guides focus on hazard identification, storage recommendations, and regulatory compliance. For instance, containers specify CAS number 7305-71-7, safety pictograms, and proper storage conditions, reminding anyone handling the material to respect its lineage as both a valuable tool and a potential hazard.
Reliable access to 2-amino-4-methylthiazole often maps back to classical synthetic methods. A familiar method, rooted in experience, joins alpha-haloketones like 2-chloro-3-butanone with thiourea under reflux. The mixture spends a decent amount of time heating in ethanol or another suitable solvent, after which work-up and crystallization yield the finished compound. This route supports large-scale applications as well as smaller lab needs. Some researchers experiment with greener or one-pot variations, coaxing improved yields by adjusting temperature, solvents, or using alternative bases—efforts that hint at a future where sustainability might sit alongside reliability.
2-Amino-4-methylthiazole acts as an agile participant in a variety of chemical transformations. The amino position opens the door for acylation, sulfonation, and condensation—functional changes that build the backbone of other organic molecules or potential drug candidates. Electrophilic substitution at the thiazole ring lets chemists install other groups at the available positions, modifying biological responses or tuning chemical reactivity. Occasionally, even its methyl group becomes a lever for further elaboration through oxidation or halogenation. In my own projects, the adaptability of this molecule offered shortcuts in multi-step syntheses—shortcuts that sometimes spelled the difference between success and another month in the lab.
In catalogs and journals, 2-amino-4-methylthiazole wears several aliases. Names like 4-methyl-2-aminothiazole, 2-amino-4-methyl-1,3-thiazole, and even less common identifiers pepper scientific papers. Some companies use their own product codes for inventory, but the CAS number remains the surest way to avoid confusion. This chorus of names often complicates searches for literature or purchase orders; I’ve learned to check spellings and codes twice before placing an order or quoting a synthesis to colleagues.
Handling 2-amino-4-methylthiazole means paying close attention to established safety guidelines. Workers need gloves, goggles, and good ventilation—not optional, but expected practice. Material safety data sheets note that dust and vapors can irritate eyes, skin, or airways. It rarely draws comparisons to the more notorious hazardous chemicals, but the possibility of harmful effects means storage requires tightly sealed containers, kept well away from incompatible substances such as strong oxidizers or acids. Training and written procedures keep workplaces safe. I’ve seen firsthand how simple protocols—like careful weighing and immediate cleanup—sidestep unnecessary accidents or exposure scares.
The biggest footprint of 2-amino-4-methylthiazole sits inside the pharmaceutical world. It often gets called upon as a precursor to drugs with anti-inflammatory, antibacterial, or antifungal activity. The heterocyclic ring shares structural traits with plenty of candidate molecules for targeted treatments, including kinase inhibitors and enzyme antagonists. Its chemistry also serves agrochemistry, where it finds use in products protecting crops or enhancing yields. Analytical chemistry, biotechnology, and material science have explored its behavior, though these fields don't command the same commercial significance as the life sciences. Any researcher after a versatile, compact core for molecule building quickly turns to 2-amino-4-methylthiazole for its welcoming reactivity and solubility.
In pharmaceutical circles, interest remains intense. R&D groups chase after analogs of established drugs, swapping out rings and groups to uncover new properties. 2-Amino-4-methylthiazole ends up at the center of many lead optimization campaigns, helping shape compounds aimed at tougher bacterial strains or drug-resistant fungi. Some researchers head in the direction of materials science, where thiazole derivatives—sometimes decorated with extra groups—lend conductive or fluorescent properties for sensors or organic electronics. Each new project borrows a bit from its chemical heritage, and sometimes, an unexpected property emerges as research unspools. The push toward green chemistry is driving new syntheses that trim waste and skip toxic solvents, aiming to keep the benefits without hiking up the costs or environmental impacts.
Toxicity data stay limited compared to more commonly used drugs or solvents, but published reports suggest moderate oral toxicity in animal models, raising caution flags for chronic exposure. Studies flag the compound as a potential skin and eye irritant, and its metabolic fate still invites investigation. Internally, I’ve seen teams take a conservative approach—using exhaust ventilation and single-use consumables, then running extra analyses to track any metabolites or degradation products. The point is always to keep exposure below recognized thresholds, especially for processes involving larger batches.
Looking ahead, 2-amino-4-methylthiazole sits at an interesting crossroads. The continued demand for new pharmaceuticals ensures heavy attention remains fixed on its modifications and analogs. The drive for sustainable chemistry will push researchers to invent less wasteful synthetic routes, possibly harnessing biocatalysis or solvent-free reactions. Advances in computational chemistry allow teams to model new derivatives, potentially predicting drug-like properties before any glassware ever gets touched. With regulatory scrutiny becoming sharper, suppliers may need to develop formulations and packaging that cut risks while offering longer shelf life. The compound's basic structure will keep playing a role in both medicine and materials, reminding every chemist or technician of the importance of careful handling, creative research, and reliable supply chains.
A lot gets wrapped into a molecule’s formula, sometimes more than folks realize. For 2-Amino-4-Methylthiazole, the molecular formula is C4H6N2S. That string of letters and numbers offers up a blueprint for a compound involved in things way beyond the chemistry classroom. There’s something satisfying about spelling it out: four carbon atoms, six hydrogens, two nitrogens, one sulfur. You look at that mix and start seeing the possibilities in pharmaceuticals, dyes, and so much more.
Nobody likes reciting molecular formulas for the fun of it. Once you see the corners this molecule touches, though, that formula matters. Thiazole rings have a reputation in the world of drug development. They show up in antibiotics, in antifungal agents, in lab-made flavors, rattling around in things most people never notice. The “amino” part opens more paths, allowing chemists to hang other groups on that base ring, building up something new, something useful.
Take a walk through drug discovery history and you’ll notice compounds shaped a bit like this one. Sulfa drugs, thiazole-based agents—they saved lives. Sometimes, basic research into small molecules like 2-Amino-4-Methylthiazole lights the way for something no one saw coming. When a chemist looks at C4H6N2S, it’s kind of like reading a map. They see roads leading off in all directions: new reactions, better yields, different therapeutic options.
Ignoring details about the structure will trip you up quick in the lab. Student or seasoned scientist, you work with what you know. The arrangement of that methyl at position 4 and the amino at position 2 affects how the molecule reacts. Reactivity changes shape and function. Modifying one atom throws the balance off, sometimes landing you far away from what you meant to achieve.
In my time helping recent chemistry grads, I’ve watched simple mistakes—a swapped number, a missing atom—slow an otherwise promising project. Getting the formula right isn’t trivia. It’s safety, accuracy, and progress all bundled together. Companies synthesizing building blocks for medicines keep close tabs on these molecular details for that very reason.
People working outside labs might shrug at molecular formulas, thinking they belong in textbooks. I get that. Tucked inside the formula, though, are hints about how thiazoles can fight infections or boost crops or flavor food. There’s value in knowing the basic setup. High school students and cutting-edge chemists both rely on these combinations.
Problems crop up when these compounds escape into the wrong places. Unintentional release of building blocks like this can foul up water, expose workers, or disrupt ecosystems. It’s on us to track and respect the information, making sure everyone from a teacher to an industrial producer handles it right.
Big discoveries start small. Recognizing the structure and importance of molecules like 2-Amino-4-Methylthiazole helps learners connect the dots between classroom theory and industrial or medical practice. Industry leaders could step up by making chemical data clearer to those who don’t have years of training. Colleges should let students build real-life skills: practicing with actual compounds, not just diagrams.
Ultimately, precision and respect for what goes into the formula protect people and drive innovation. Behind every string of chemical symbols, a vast web of research, application, and responsibility follows.
Not every chemical grabs headlines, but 2-Amino-4-Methylthiazole turns quiet influence into daily relevance. Folks who work in pharmaceuticals or crop protection know the value of compounds like this. I remember walking through my first chemistry internship, shelves lined with tiny bottles marked with names longer than grocery lists. Even in those plain glass vials, you could find building blocks for life-changing products.
If you’ve ever taken prescription medication, there’s a good chance there’s a thiazole ring somewhere in manufacturing history. 2-Amino-4-Methylthiazole works well for chemists designing the scaffolding of new drugs. Its thiazole structure pops up during the early stages when drug companies hunt for molecules with good biological activity—think antibiotics, anti-inflammatories, and sometimes cancer drug research. Rather than churning out one pill after another, labs use this compound to patch together pieces of promising medicine. Pfizer and Merck have looked at similar molecules in the development of new compounds.
Growth in the field doesn’t just show up in new seeds—chemistry leads the way in plant protection, and here’s where 2-Amino-4-Methylthiazole proves its worth. Anyone who’s tried to keep tomatoes alive during a wet summer knows the pain of crop diseases. Thiazole derivatives sneak into the core of several fungicides and herbicides. The compound makes it easier to tweak formulas and test combinations that protect crops from blights and insects, with research stretching from the Midwest's soybean fields to greenhouses across Europe. Farmers may never read the ingredient list, but their annual harvest often depends on discoveries that grow in a beaker.
Color helps us make sense of the world—whether glancing at litmus paper during a science fair or sorting through clothes. Chemical companies sometimes turn to 2-Amino-4-Methylthiazole to develop dyes and pigments that last longer and perform better under light and heat. Its structure lets scientists play with shades and brightness, a trick that shows up in everything from school chemistry sets to quality-control strips in food production. On the fancy end, dress clothes and specialty fabrics get their pop from molecules built with the help of this compound.
Food scientists love a challenge, and flavor work owes much to thiazole chemistry. This compound can mimic or enhance savory tastes in culinary products, bringing out that extra note in broths or snack seasonings. Small tweaks in the lab make flavors that stick around, cutting costs for big food companies and boosting taste consistency. On the synthesis side, chemists reach for 2-Amino-4-Methylthiazole to build new molecules for research. It acts like a puzzle piece that fits in just the right spot, helping researchers create everything from anti-bacterial coatings to electronics chemicals.
Sourcing and handling any specialized molecule calls for care. Fake or low-quality batches can mess up results, raising costs or causing headaches for manufacturers. Regulations push chemical suppliers to ensure safety and purity—a step that matters in medicines and foods. Environmental concerns nudge the industry toward greener methods, like swapping harsh solvents for milder alternatives or recycling waste during synthesis. Direct connections between lab and life mean that any improvement in efficiency, yield, or safety touches a wide circle—from scientists and factory workers to people at the pharmacy counter or grocery aisle. Here, innovation means not just new molecules, but better ways of bringing them to the frontlines of daily living.
Working in labs, your relationship with chemicals depends on trust. Trust that your chemicals stay stable and do the job they’re meant for. I’ve seen more than one researcher lose weeks of effort because a simple storage slip-up ruined a whole stash of reagent. 2-Amino-4-Methylthiazole isn’t some rare mystery substance, but treating it without care invites trouble.
Chemists will tell you: thiazoles in general don’t appreciate heat, moisture, or direct sunlight. 2-Amino-4-Methylthiazole likes the shade, a steady room temperature, and a tightly sealed jar. Humidity can sneak in, clump up your powder, or trigger slow breakdown you might not spot until it’s too late. Every label says “store in a cool, dry place”—not quirky advice, just hard-won wisdom. I remember old stock jammed on a shelf right above the radiator. One summer, the jar fused shut, and the stuff inside yellowed and stank. Ended up dumping the whole thing out.
Keep the room temperature between 2°C and 8°C if you can manage it. At universities and some industry labs, a dedicated chemical fridge makes a difference. No food or drink ever belongs inside. Avoid freezing unless your MSDS specifically calls for it. Freezing and thawing cycles encourage condensation. Moisture, even a whiff, can make these powders clump up, degrade, or sometimes form new byproducts that don’t belong there.
Sealing goes beyond twisting on the cap. I double-bag messy thiazoles in polyethylene and label everything. Don’t ever pop a desiccant bag in there “just in case” unless you checked it won’t react. Silica gel can sometimes react with certain organic compounds. Use a clean, dry environment, and separate your thiazoles from strong acids, bases, and oxidizers. Fumes from those can travel. I once stacked a batch of 2-Amino-4-Methylthiazole right next to a bottle of strong ammonia. Left unchecked, thiazole “fumes” found their way over, and next thing I knew, the whole section had to be cleaned out.
Old habits die hard, but there’s no excuse for sloppy labeling. Even with the best storage, nothing lasts forever. I keep a note of the opening date, expiry, and any signs of color change. 2-Amino-4-Methylthiazole starts off as a pale yellow powder. If it clumps, darkens, or smells sharper than usual, it’s safest to get a new supply. Cutting corners on labeling leads to confusion—just one wrong scoop into a flask can wreck results or waste valuable time.
I’ve worked in places where space ran tight and money ran tighter. It’s tempting to toss all your solids on one shelf. Resist that urge. Cheap plastic bins for organics, acids, bases, and oxidizers won’t cost much but save plenty of headaches. I’d rather spend a few extra minutes now than explain why a $300 project needs a do-over.
Order small batches if you aren’t cranking through this chemical every month. Cut back on waste, and keep things fresher. Never use leftover material if it sat open for weeks in poor conditions. Share best practices across the team so everyone brings their supply habits up to par. A small routine around storage turns into big savings in safety and productivity down the line.
Every chemical on a lab shelf tells its own story, and 2-Amino-4-Methylthiazole holds its place among the lesser-known but important ones. Often used in making pharmaceuticals and agricultural chemicals, this compound doesn’t grab headlines, but that doesn’t make it a free pass in terms of safety. The common approach can sometimes be, “If it isn’t a household name, it can’t be that risky.” That sort of thinking doesn’t fit this one.
Ask anyone who’s worked in a chemistry lab long enough—new names pop up every year, but safety basics don’t disappear. 2-Amino-4-Methylthiazole carries a noticeable risk to the skin and eyes. A careless splash may result in irritation or an allergic response. I once saw a colleague forget safety glasses while handling a similar compound, and she spent hours flushing her eyes at the eyewash station. The lesson sticks: even if the label looks innocent, it deserves respect.
Looking into toxicity, rodent studies point to moderate oral toxicity. That’s not “death by a drop,” but you wouldn’t want to eat your lunch in the same spot you just weighed this stuff out. Breathing it in could irritate the nose and throat, according to the information I’ve read, much like many fine powders or dusts in the lab.
People often look for a red flag—does a substance spark into flames, give off toxic gas, or eat through steel? In the case of 2-Amino-4-Methylthiazole, it won’t ignite the moment it leaves the bottle, but don’t get too relaxed. The right approach follows habits: gloves that fit well, eye protection you actually wear, and handling under a fume hood. The chemical isn’t so volatile you need a hazmat suit, but it doesn’t reward shortcuts either. Even handling the powder without a mask can invite trouble if you tend to brush dust off your hands or rub your eyes.
One fact that stands out: this compound ranks as a mild irritant, not a poison gas or burn risk. Still, enough exposure builds up harm over time, and environmental risks pile up if people pour leftovers down the drain. In my experience, old habits die hard—someone always feels tempted to wash away spills rather than sweep up and dispose of them as hazardous waste. That quick fix never pays in the long run.
Keeping things safe really boils down to attention and respect for small-print details. Label every container, keep substances sealed, and store them where accidental spills can’t reach the wrong places. Proper training comes into play, especially for people just starting out. In my first year, I thought I could learn safety as I went along. That lasted until a minor spill forced an entire bench to shut down for cleanup. Awareness and a willingness to ask questions make a difference.
Treating 2-Amino-4-Methylthiazole with the same mindset as other reactive chemicals—gloves, glasses, proper ventilation—pushes back against carelessness. Following storage guidelines, such as keeping it away from acids or bases, cuts down on accidents. Regular reviews of safety protocols don’t just fill checklists; they save skin, time, and even the occasional reputation.
That’s the kind of respect every chemical deserves, whether its name is famous or not.
2-Amino-4-methylthiazole pops up in labs all over, from pharmaceutical research to chemical supply catalogs. The question that often follows its mention isn't about its uses but about its purity. Many people working in synthesis—myself included at points—want to know what they’re truly getting. Most suppliers offer this compound at a minimum of 98% purity, and that number seems to stick. It's not simply pulled from thin air; it speaks to what industry has come to expect in order to keep reactions predictable, results reproducible, and safety on track.
Why 98%? Lab work can succeed or fall apart based on impurities. I learned early on that even a sliver of the wrong contaminant can ruin an otherwise perfect day at the bench. Imagine trying to isolate a product, and a stubborn impurity refuses to separate. Higher purity means fewer headaches during purification and less time spent chasing ghosts across chromatograms. Over the years, 98% became the gold standard for most applications—high enough for solid results, realistic enough for suppliers to keep costs manageable.
I've also found that people rarely look at purity in isolation. Small traces of solvents, ash, moisture, and related thiazoles tend to make up the bulk of remaining impurities in typical batches. Analytical reports, usually attached to commercial shipments, list these out in black and white. Once, I received a lot that hovered just under 97%. Even if the supplier offered a discount, I paid for it later in failed steps. There’s a reason most catalogs won’t go lower than that number unless you’re buying in bulk and don’t mind rolling the dice.
It can be easy for those not working directly with chemicals to underestimate the ripple effects of purity. Let's say a medicinal chemist wants to build a new drug scaffold. Little differences in starting material can derail activity screens or worse, trigger unexpected reactions. In routine industrial syntheses, what seems like a tiny impurity can tank a yield or introduce byproducts that don’t show up until scale-up. I’ve spoken to friends in manufacturing who’ve had entire production lots delayed simply because a supplier sent material with “off-spec” contamination. Regulatory headaches soon follow.
The stories chemists share in break rooms often come down to one lesson: shortcuts on quality wind up costing more money and time. Purity protects not just the end-user, but everyone along the chain, from raw material handler to final formulator.
Purity isn’t just an abstract benchmark. Greater transparency from suppliers helps. Certificates of analysis should always accompany shipments. It’s on the purchasing chemist to check batch numbers, review HPLC or GC traces, and push back when things don’t add up. Labs should establish a protocol for routine quality checks, not just for the rare big-ticket items but for everyday reagents like this compound.
Improving relationships with suppliers also matters. I’ve found that having open lines of communication and clear expectations keeps both parties honest. If labs and companies keep demanding top-notch product and follow up on lapses, market standards gradually rise across the board. For 2-amino-4-methylthiazole and a thousand other chemicals, it isn’t just about purity—it’s about trust earned, lesson by hard lesson.
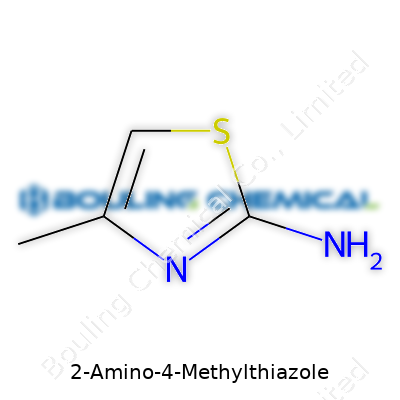
| Names | |
| Preferred IUPAC name | 5-methyl-1,3-thiazol-2-amine |
| Other names |
2-Amino-4-methyl-1,3-thiazole 2-Amino-4-methylthiazole 4-Methyl-2-aminothiazole 4-Methylthiazol-2-amine |
| Pronunciation | /tuː-əˈmiːnoʊ fɔːr ˈmɛθəl θaɪˈæzoʊl/ |
| Identifiers | |
| CAS Number | 1603-91-4 |
| 3D model (JSmol) | `3D:14 N1C=CSC1C JSmol Model ` |
| Beilstein Reference | 120924 |
| ChEBI | CHEBI:20593 |
| ChEMBL | CHEMBL12343 |
| ChemSpider | 12328 |
| DrugBank | DB08243 |
| ECHA InfoCard | 100.018.213 |
| EC Number | 217-673-4 |
| Gmelin Reference | 70208 |
| KEGG | C06476 |
| MeSH | D01899 |
| PubChem CID | 86618 |
| RTECS number | XZ3150000 |
| UNII | UVA782F5Y8 |
| UN number | Not regulated |
| Properties | |
| Chemical formula | C4H6N2S |
| Molar mass | 114.17 g/mol |
| Appearance | Light yellow to brown crystalline powder |
| Odor | amine-like |
| Density | 1.18 g/cm³ |
| Solubility in water | Soluble |
| log P | 0.3 |
| Vapor pressure | 0.159 mmHg (25°C) |
| Acidity (pKa) | 6.0 |
| Basicity (pKb) | 6.36 |
| Magnetic susceptibility (χ) | -64.5·10^-6 cm³/mol |
| Refractive index (nD) | 1.5900 |
| Dipole moment | 2.96 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 146.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -12.3 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3687 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302 + H312 + H332 |
| Precautionary statements | P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362+P364, P405, P501 |
| Flash point | 88°C |
| Autoignition temperature | 355 °C |
| Lethal dose or concentration | LD50 oral rat 2000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 1310 mg/kg |
| NIOSH | MF9275000 |
| PEL (Permissible) | Not Established |
| REL (Recommended) | REL: 5 mg/m³ |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Thiazole 2-Aminothiazole 4-Methylthiazole Benzothiazole 2-Mercaptothiazole |