Back in the early days of pyrazine chemistry, the creative spark fueling brominated derivatives came from a drive to improve building blocks for organic synthesis. Chemists, especially those focused on heterocyclic technology, started shaping molecules like 2-Amino-3,5-Dibromopyrazine to address the demands of pharmaceuticals and agricultural chemicals. This development gained steam alongside advances in halogenation methods during the latter half of the twentieth century. Labs started tuning procedures, learning that structural tweaks on the pyrazine core could spark new reactions and repair old roadblocks in synthetic routes. Today, this compound connects past innovation with industry needs, allowing research and manufacturing teams to dig deeper into previously inaccessible targets.
The solid known as 2-Amino-3,5-Dibromopyrazine stands among the go-to intermediates for people seeking versatility. Its fused pyrazine ring, locked with two bromine atoms at positions three and five, brings reactivity and specificity. Tasks like making pharmaceutical ingredients or prepping specialty chemicals rely on these properties. Labs leverage this compound to cut down synthetic steps and open up new paths to derivatives, all thanks to the predictable way the amino and bromo positions behave. For custom molecule builders, a supply of this dibromo variant gives a steady hand in guiding functional group transformations.
Anyone who’s handled 2-Amino-3,5-Dibromopyrazine recognizes it by its light beige crystals. The melting point often hovers around 175-180°C, ringing in with stability that’s suited to classic storage conditions. Solubility trends follow the pyrazine backbone: it's only slightly soluble in water, but dissolves better in polar organic solvents such as DMF or DMSO. Chemically, the presence of amino and dibromo groups introduces a host of reactivity avenues, including ready halogen exchange and coupling steps. The firm structure holds up under air, but the molecule’s halogens mean users need to think through compatibility with strong nucleophiles and reducing agents.
Quality control teams take these standards seriously. Buyers expect an assay above 98% purity, and documentation should state any trace impurities, lingering solvents, or water content. Crystal morphology can shift based on synthesis pathway, so vendors often specify texture and color to guide recipients. Safety labeling covers the hazards tied to organic bromides: respiratory and eye precautions, gloves for direct handling, and safe wastewater management protocols. Certificates of Analysis should clearly present batch-specific details— from melting point to elemental analysis— ensuring downstream applications won’t get sidetracked by unexpected side products. These checks boost both regulatory confidence and research reliability.
Chemists lean on two main prep routes. The first starts with pyrazine rings, using selective bromination and then directing an amino group to the two-position via nucleophilic substitution or amination. Older procedures relied on direct bromination of aminopyrazine, but more controlled approaches, like stepwise halogenation with protecting groups, have improved clean-up and yields. Labs try to dial down byproducts and streamline purification, often pulling on chromatography or recrystallization from polar solvents. Trends in green chemistry encourage swapping harsh brominating reagents for milder agents, but the challenge lies in balancing efficiency and low-residue output.
The real power behind 2-Amino-3,5-Dibromopyrazine shows up in its reactivity. Suzuki and Buchwald-Hartwig couplings find fertile ground on those two bromo positions, opening doors to biaryl pyrazines and diverse amino-pyrazine libraries. The amino group steps up for acylation, diazotization, or further substitution, often streamlining construction of more complex nitrogen-containing scaffolds. Medicinal chemists bank on this site as a launchpad for making kinase inhibitors or antibiotics, where each transformation can fine-tune biological activity. For materials scientists, those halogens let users bolt on aryl or alkynyl groups without rewriting whole synthetic plans.
In catalogs and research papers, product listings rotate through various synonyms. Some call it 3,5-Dibromo-2-pyrazinamine. CAS databases record a cluster of registry identifiers, letting different suppliers align their records and SDS documents. Literature may shorthand the compound as Dibromoaminopyrazine or slip into systematic IUPAC nomenclature depending on the publication. These names point to the same core, adding context based on region, supplier, or end-use sector— but each label always relates back to the critical theme: a pyrazine with halogens at three and five, and an amino group holding the fort at the second position.
Anyone in the lab knows chemical safety isn’t a box to tick. Workers must use gloves, chemical goggles, and fume hoods to steer clear of inhalation or skin contact hazards tied to brominated organics. Spillage needs swift clean-up— containment, deactivation, and careful disposal via hazardous waste systems, never down the drain. Long-term exposure studies recommend air monitoring, especially in synthesis-intensive sites. Handling protocols include explicit instructions on segregating pyrazine derivatives from strong acids, oxidizers, and reducing agents, keeping workbenches tidy and reactions predictable. Records on air monitoring, waste output, and incident logs matter for both safety audits and environmental compliance.
The scope of applications stays broad. Pharmaceutical teams pull this molecule for small-molecule library synthesis, searching for next-generation drug scaffolds. Agrochemical projects use it to design new fungicides or herbicides, building on the pyrazine core. Dye manufacturers have used related dibromopyrazines to introduce color fastness and stability in textile products. In materials science, the dibromo handles enable tailored polymer architectures and advanced functional materials, putting this compound on the roster for R&D teams exploring electronic and photonic opportunities. Its underlying structure supports these roles by bridging established and emergent technical needs.
Research into new synthetic methodologies depends on tools like 2-Amino-3,5-Dibromopyrazine, especially as more efficient cross-coupling protocols emerge. Medicinal chemists searching for kinase inhibitors have dug into pyrazine libraries, looking for selectivity in cancer treatment. Process chemists test greener brominating reagent alternatives to keep production sustainable. Material scientists want to fine-tune pyrazine derivatives for better electronic response in organic semiconductors. These projects benefit from reliable intermediates, letting each lab test new ideas, build critical structure-activity relationships, and aim for greener, higher-yield synthesis that cuts waste and improves access to new compounds.
Toxicologists take brominated aromatics seriously, mindful that halogens can amplify bioaccumulation or ecological persistence. Results for 2-Amino-3,5-Dibromopyrazine itself suggest moderate toxicity, especially with repeated ingestion or skin contact. Eye and skin irritation top the usual safety concerns, triggering global harmonized system warnings in most regulatory environments. Early environmental fate assessments suggest low water solubility curbs risk of rapid aquatic spread, but safe disposal into properly designed incineration or chemical treatment systems makes the difference. Regular research updates keep tabs on new findings in occupational health, informing guidelines for personal and environmental protection down the road.
Looking ahead, the role of 2-Amino-3,5-Dibromopyrazine isn’t set to shrink. Market growth for targeted therapeutics and agricultural chemicals keeps it in demand. Teams working on green chemistry want milder, safer bromination procedures, nudging the field toward less waste and cleaner processes. The rise of machine-learning-based molecule discovery puts a premium on reliable building blocks like this one. Material science’s hunger for custom, functional heterocycles means researchers will keep returning to the dibromopyrazine motif for years to come, driven by both necessity and the promise of new discoveries.
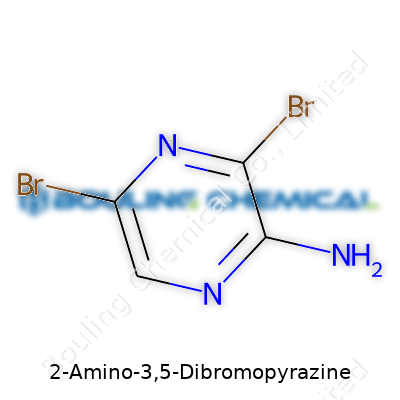
The structure of 2-Amino-3,5-dibromopyrazine comes from the pyrazine ring. Pyrazine itself holds two nitrogen atoms at opposite corners of a six-membered ring. Here we add some key groups: an amino group at position two, and bromine atoms at positions three and five. The chemical formula comes out as C4H3Br2N3. Each part adds something distinct – carbon and nitrogen build the ring, bromine brings heft and reactivity, and the amino group tips things in a specific direction for how this compound reacts with others.
It’s easy to focus just on numbers and letters in a formula, but the shape and bonding mean a lot. People in pharmaceutical labs watch for these halogenated molecules because the bromine atoms often change how the compound acts in the body. A molecule with bromine isn’t just heavier; it often survives longer in chemical reactions and sometimes in the liver too before breaking down. Tweaking a small thing like one atom can totally flip how a molecule treats bacteria, cancer cells, or inflammation. Some brominated pyrazines show real promise in antimicrobial studies. My old lab used to run these head-to-head against established antibiotics, and just a switch of position bumped up effectiveness. It’s powerful stuff, and the roots of those changes sit clearly in formulas like this one.
In my own experience, the moment "dibromo" pops up in a compound name, safety hats go on. Brominated chemicals don’t always break down easily. They can linger in soil or water and take long chains of bacteria years to even dent. That’s why proper disposal and traceability stay crucial in both small college teaching labs and big commercial plants. Some countries crank rules tighter every year after finding accidental buildups near manufacturing sites. Scientists and chemical engineers work hard to find greener or more biodegradable alternatives, but progress still needs to speed up in many parts of the world.
Pyrazine rings stay useful in many designer drugs, dyes, and agricultural chemicals. For people running this synthesis, it pays to double check sourcing. Clean bromine reagents, strict ventilation, and solid training lower danger – I remember a spill during my time training younger chemists; nobody got hurt, but it’s a wakeup that unpredictable things happen if you slack on safety.
On the environmental front, green chemistry offers tools to cut down the footprint. Some teams use flow chemistry or look for biological catalysts that do the job cleaner and with fewer byproducts. Students now learn to design reactions that plan for waste and safety from day one. Knowing the formula is only the start – understanding impact and real-world utility builds the bridge between theory and safe practice. That’s where researchers, teachers, and regulators all find common ground, using each bit of data—and each formula like C4H3Br2N3—to make better decisions for science and society.
For folks who spend time in research labs—or even follow the steady march of innovation in medicine—2-Amino-3,5-Dibromopyrazine stands out as a building block. Chemists reach for this compound because its structure helps design molecules that fight off disease. The arrangement of the amino group and two bromine atoms gives it a special talent for shaping new chemical entities, especially in early drug discovery.
In real drug labs, most work happens in tiny vials. Teams focus on tweaking molecules to get better activity or fix side effects. This pyrazine derivative often crops up as a starting point during the hunt for novel antiviral and anticancer agents. Its rigid ring, dressed with reactive sites, lets chemists snap new parts onto it. That flexibility matters when you’re looking for molecules that can hit tough targets, like stubborn viral enzymes or cancer growth factors. If you scan recent papers in medicinal chemistry, you’ll see countless late-stage compounds with this very framework anchoring their design.
Applications spread far beyond health care. Materials researchers lean on this molecule thanks to its chemical stability and convenient bromine atoms. These features give manufacturers a way to build more complex systems, such as organic electronics. In these projects, small tweaks to the base structure lead to new polymers and film coatings, which show up in flexible displays and next-gen solar panels.
Brominated pyrazines lend themselves to more than just hardware. The electronics industry values intermediates that plug directly into synthetic workflows. With this compound, each team along the way can make predictable changes without starting from scratch. Folks working to develop conductive materials or test the limits of organic semiconductors know this backbone opens up those possibilities, letting new tech leap off the drawing board.
Diagnostics labs also turn to 2-Amino-3,5-Dibromopyrazine for its stability and reactivity. In my own bench work, I’ve watched how compounds like this help create diagnostic dyes. Add a few tweaks, and you get probes that stick to target proteins in blood samples. For hospitals and research centers, these probes sharpens everything from infection detection to cancer screening.
There’s a comfort in reaching for chemicals with a reputation for reliability. Scientists know this molecule offers a predictable pathway as they build and test new analytical methods. For every big breakthrough, countless experiments depend on dependable intermediates like this one.
Production of specialty chemicals isn’t always simple—or clean. In my conversations with process engineers, waste management and worker safety come up nearly as often as yield optimization. Brominated chemicals, while useful, demand extra steps for waste disposal. Bringing that process in line with stricter environmental standards means investing in cleaner synthesis routes. Green chemistry offers solutions: using water-based solvents and recycling bromine.
As demand grows in pharma and electronics, companies have a chance to lead with transparency. Open publications about both safety data and greener production methods would help others improve practices too. The chemical industry can move forward without sacrificing either innovation or responsibility to both lab workers and the public.
Every lab bench story starts with one question: how clean is the stuff in the vial? 2-Amino-3,5-Dibromopyrazine has been around in research circles for a while, mostly because it acts as a useful starting point for more complex compounds. Purity makes the difference between results you can trust and results that could waste days or months of work.
A chemist who’s spent hours puzzling over inconsistent results knows that even tiny amounts of impurity can mess up entire syntheses. Research-grade chemicals usually list 97% or better purity. But real testing always shows: those last two or three percent shape everything. In the case of 2-Amino-3,5-Dibromopyrazine, most suppliers today quote purity levels between 97% and just above 99%. For most synthetic or medicinal tasks, 98% works fine, but if you plan on running sensitive bioactivity screens or making active pharmaceutical intermediates, each decimal point starts to matter a lot more.
Raw numbers on the spec sheet never tell the whole story. Purity, by lab convention, usually counts visible contamination, trace solvents, moisture content, and unreacted starting materials. The industry uses high-performance liquid chromatography (HPLC), nuclear magnetic resonance (NMR), and mass spectrometry to check these things. If the certificate of analysis reads 98%, that doesn’t always describe what the leftovers are. Some impurities slip in during manufacturing and might be close relatives of the main molecule—compounds that react in unexpected ways.
Living through graduate school, I remember the pain of working up a reaction, drying down my pyrazine product, and finding something just off in the NMR. Even one fraction below 99% caused cascade problems in multi-step syntheses. I’ve seen teams spend weeks chasing a minor contaminant that kept fouling up our final target. Anyone repeating old syntheses can tell stories like this.
Chemists learn to never trust a label blindly. They read the certificate of analysis before every order and sometimes run their own purity checks before putting a product to use. It’s not about lack of trust, it’s survival in a world where a hidden contaminant can drain your budget. Reliable suppliers spell out their testing methods. A real certificate describes HPLC traces, NMR results, residual solvent, and water by Karl Fischer titration. The best labs keep things transparent, answering customer questions without delay.
One time, I asked a vendor why a 98% sample kept giving odd spots in TLC. After back-and-forth, we found out the batch contained a positional isomer. It wasn’t listed in the certificate, and they replaced the lot, but only after weeks of downtime. That’s why vetting both the product and the provider really matters. Only buy from places that answer your questions right away and send detailed certificates with every order.
To address persistent purity issues, there needs to be more widespread use of modern analysis and more transparent reporting. Chemical resellers could add photographs of actual spectra, not just the number summary. Customers should ask more questions and insist on a full breakdown, not just a percentage. Some teams might need to purify the chemical further with their own chromatography before using it in a sensitive reaction.
In the end, asking about purity isn’t just ticking a box for compliance. It’s protecting your project and your time. If something feels off, it often is. Good lab practice means double-checking purity—and pushing suppliers to do the same.
Growing up in a household where mishandled paint thinners or household chemicals led to cringe-worthy cleanups, I learned early on how small details make the difference between a safe shelf and an expensive disaster. In the lab, storage isn’t just a matter of convenience—it can make or break the quality, safety, and value of a chemical like 2-Amino-3,5-Dibromopyrazine. This pyrazine derivative, with its bromo and amino groups, brings both promise and risk to research and production. A few small missteps can dull purity, lead to unwanted side products, or even turn a valuable bottle into hazardous waste.
Glass stands strong as the top choice for holding 2-Amino-3,5-Dibromopyrazine. Glass doesn’t react with the molecule, and it doesn’t leach plastics or metals into this sensitive compound. The tight seal of a screw-cap bottle helps keep air and moisture out. A desiccator adds an extra layer of protection, especially in regions where humidity creeps above 50%. I’ve seen well-intentioned labs store fine chemicals in reused plastic containers. In time, trace impurities from those containers quietly creep in, compromising research results and sometimes bringing strong, unusual odors.
Room temperature in most controlled settings keeps 2-Amino-3,5-Dibromopyrazine steady. Direct sunlight or heat jumps the pace of degradation, and too much cold can draw moisture once containers open and close with varying temperatures. A stable environment—think of what feels comfortable for a human in a lab coat—rarely disappoints. I once watched a fridge overloaded with reagents break into chaos after a single thermometer stopped working. There’s wisdom in checking temperature logs and not crowding shelves.
Water vapor inches in invisibly. Even if the chemical looks fine, unseen moisture works away at the amine group, encouraging hydrolysis or complex byproducts. Silica gel packets, changed out every few months, go a long way. It doesn’t take fancy tools—just the habit of swapping them before the indicator beads lose color. These small packets saved us more than once from tossing otherwise expensive material.
Pyrazines and their derivatives can shift with exposure to oxygen and light, especially bright fluorescent bulbs often found in older labs. I keep amber bottles and opaque boxes on hand for these jobs, away from direct overhead lights. In our lab, we mark storage dates and track the opening and closing of each container. Regular checks catch clumps and color changes long before they turn into research roadblocks.
Simple habits keep confusion at bay. Clear labels with chemical name, date received, and last opened beat messy mystery jars every time. I keep inventory logs with batch numbers, spotting trends in stability or trouble with suppliers. Rotating stock avoids reaching for a bottle that’s been collecting dust for years.
Lab coats, gloves, and splash-proof safety glasses make handling routine. It’s tempting to skip these steps on busy days, but human health always ranks first. I once saw a colleague save minutes but lose days to a hand rash. Dust masks help contain loose powders and avoid accidents when weighing the material.
Digital temperature and humidity loggers, with alerts for sudden swings, offer peace of mind for those handling kilo scales or high-sensitivity work. Investing in proper shelving, away from sources of vibration and chemical spills, keeps storage safe and organized.
Taking the time to store 2-Amino-3,5-Dibromopyrazine right boils down to respecting both the molecule and the people around it. In the end, the few extra steps pay off in safety, savings, and reliable science.
A certificate of analysis isn’t just another sheet shuffled in with a shipment. It’s the only document telling you, in black and white, what’s inside the chemical bottle. In labs, I’ve seen people trust labels and catalog numbers, only to find the product didn’t live up to expectations. If you get 2-Amino-3,5-Dibromopyrazine without any supporting data, you end up gambling with important research, industrial runs, and funding.
During work on projects demanding high-purity ingredients, uncertainty has ruined days of prep and put tight budgets on the line. Not all suppliers care enough to send a COA by default. Those who skip it might not test every lot, or worse — might not have much to tell you about what they’re selling. If you’re buying for research or manufacturing, skipping the COA is risky. The best labs treat a product without a COA much like unlabelled food in the fridge: it could be fine, but no one trusts it.
It’s not just about obtaining some paperwork. A solid COA lays out the results of tests like NMR, melting point, purity by HPLC or GC, moisture levels, and sometimes traces of impurities. Without confirmation from the supplier, you can’t prove the identity and purity of 2-Amino-3,5-Dibromopyrazine. Everyone I know in research expects at least that bare minimum.
Faking test results isn’t unheard of. An article in the Journal of Chemical Education once detailed cases where companies issued generic or outdated COAs just to move stock. Plenty of experienced chemists now call or email suppliers directly and check that the COA comes with each batch — not just a vague promise posted online. An official-looking PDF isn’t enough. The data should reference your specific lot or batch number, not some standard value copied from a database.
A reliable supply chain rests on transparency. Without a trail from raw materials to delivered sample, quality claims become questionable. If a chemical supplier refuses to send a COA, that reveals plenty about their standards. I once dealt with a supplier who shrugged off the request, only offering a “typical analysis.” They lost the order, and a long-term relationship evaporated. Trust is earned, not granted.
In regulated spaces like pharmaceuticals, a missing COA can mean audit headaches and recalls. Even in academic settings, journals and grants demand clear evidence about what compounds researchers use. If you publish with unknown or unverified materials, you’ll struggle to convince anyone you know what happened in your experiments. That doubt can set a career back months or years.
Buyers shouldn’t feel shy about demanding COAs — for every purchase, not just large orders. Industry-wide standards need regular updates to keep up with new testing technologies and tighter regulations. Getting this right benefits everyone. It speeds up troubleshooting, keeps users safe, and allows buyers to hold suppliers accountable.
Unless chemical companies face pressure from buyers, some will continue rolling the dice with quality. Share experiences, ask tough questions before buying, and return products that don’t meet your standards. Everyone wins when trust in supply goes up. The piece of paper called a COA isn’t just bureaucracy — it’s a line between safety and disaster.
| Names | |
| Preferred IUPAC name | 2-amino-3,5-dibromopyrazine |
| Other names |
3,5-Dibromopyrazin-2-amine 2-Amino-3,5-dibromo-1,4-diazine |
| Pronunciation | /tuː-əˈmiːnəʊ-ˌθriːˈfaɪv-daɪˈbroʊmoʊ-paɪˈreɪziːn/ |
| Identifiers | |
| CAS Number | 6939-23-1 |
| Beilstein Reference | 631619 |
| ChEBI | CHEBI:91215 |
| ChEMBL | CHEMBL138973 |
| ChemSpider | 87270 |
| DrugBank | DB08342 |
| ECHA InfoCard | 03d17f43-5f2a-4ff3-b77c-2d5f06c89e11 |
| EC Number | 876-05-1 |
| Gmelin Reference | 702430 |
| KEGG | C19114 |
| MeSH | D020123 |
| PubChem CID | 11764601 |
| RTECS number | XU6446000 |
| UNII | 8H7M5RNH8F |
| UN number | Not regulated as hazardous for transport |
| CompTox Dashboard (EPA) | DJ5LQW5OV7 |
| Properties | |
| Chemical formula | C4H3Br2N3 |
| Molar mass | 292.916 g/mol |
| Appearance | Off-white to light brown solid |
| Odor | Odorless |
| Density | 2.33 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 0.1 |
| Vapor pressure | 2.19E-4 mmHg at 25°C |
| Acidity (pKa) | 5.9 |
| Basicity (pKb) | 5.1 |
| Magnetic susceptibility (χ) | -91.1·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.752 |
| Dipole moment | 2.75 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 317.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -25.6 kJ/mol |
| Hazards | |
| Main hazards | H315, H319, H335 |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P280-P305+P351+P338-P337+P313 |
| NFPA 704 (fire diamond) | 2-2-0 |
| Flash point | 103.7°C |
| LD50 (median dose) | LD50 (median dose): >2000 mg/kg (rat, oral) |
| NIOSH | DT8225000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 20 mg |
| Related compounds | |
| Related compounds |
3,5-Dibromopyrazin-2-amine 2-Amino-5-bromopyrazine 2-Amino-3-bromopyrazine 2-Aminopyrazine 3,5-Dibromopyrazine |