People have been working with thiophene compounds since the early days of organic chemistry. Back in the 19th century, researchers stumbled upon thiophene while tinkering with coal tar, and it didn’t take too long before they discovered that the family of thiophenes could be tweaked in countless ways. The addition of an acetyl group at the second position gave us 2-Acetylthiophene, giving rise to a compound that would catch the interest of both the fragrance and pharmaceutical sectors. Early experiments with reactions like Friedel–Crafts acylation led chemists down the path to synthesize this molecule in a straightforward fashion using relatively simple reagents, and that accessibility opened doors for more widespread adoption. It’s impressive how such discoveries, often driven by curiosity and resourcefulness rather than massive budgets, set the stage for real advancements in medicine and materials science.
2-Acetylthiophene stands out as a colorless to pale yellow liquid with a distinct, slightly nutty aroma reminding some people of popcorn or roasted grain. The popularity of this chemical often begins in small-scale labs and ends up in large-scale industrial vats, thanks to its versatility—not just in research, but in direct applications like perfume and food additives. Ask anyone who's spent time in a fragrance laboratory, and they'll tell you that compounds like this one are worth their weight for the nuance they add to blends. Although its utility goes far beyond aroma, most of its major markets lean on that spicy, slightly sulfury backbone.
Holding a molecular formula of C6H6OS and a molar mass near 126.18 g/mol, 2-Acetylthiophene boils close to 215°C and freezes around -23°C. Its low flash point, just above 99°C, means it doesn’t need as much heat before posing combustion concerns—something any bench chemist will have drilled into them. The compound isn’t terribly soluble in water, but it mixes well with organic solvents, making it handy in reactions and isolation procedures. Its refractive index sits around 1.543, offering one way to verify its presence with routine lab equipment.
Anyone buying or working with 2-Acetylthiophene needs to keep their eyes on purity. Commercial grades typically come upwards of 98%, but even fractions of a percent of residual sulfur or acetone can throw off sensitive applications like pharmaceuticals or certified flavors. Labels on barrels might list synonyms or alternative names such as 1-(Thiophen-2-yl)ethanone or Ethanone, 1-(2-thienyl)-. Labels should flag its flammability and possible irritant effects, both to skin and the respiratory tract. Establishing the right documentation—like material safety data sheets and unique identifiers (CAS number 88-15-3)—is part of the routine to keep everyone out of trouble.
The classic approach involves Friedel–Crafts acylation, mixing thiophene and acetic anhydride (sometimes acetyl chloride) with a Lewis acid like aluminum chloride. Anyone who's followed this reaction process will remember the dark, tarry mess that sometimes forms if conditions run too hot or if water sneaks into the system. Over the years, both academic and industrial chemists have tried to push down waste and bump up yields by using greener solvents, better catalysts, or continuous flow setups. Still, most labs stick with the established recipe because it works and doesn’t demand fancy equipment.
2-Acetylthiophene has a reactive carbonyl group alongside an aromatic ring, which sticks out to synthetic chemists as an invitation for further modification. The acetyl function can undergo reduction to give 2-ethylthiophene, or condensation reactions to make fragrant propenone derivatives. Bromination, halogenation, and hydrogenation open up new routes for pharmaceuticals or advanced materials. I’ve worked with modifications of this compound to spin off ligands in catalysis, and the range of resulting byproducts can either be a headache or a goldmine, depending on specificity and the patience of the lab crew.
On paperwork, 2-Acetylthiophene often goes by alternative tags. Besides the systematic 1-(thiophen-2-yl)ethanone, common listings include Ethanone, 1-(2-thienyl)-, and several shorter commercial names like Acetylthiophene or ATE. Chemists and buyers both juggle these names, so everyone in the supply chain needs to stay alert to avoid ordering the wrong isomer or getting stuck with a mismatched substitute.
Any operation that handles 2-Acetylthiophene brings housekeeping into focus due to its fire risk and possible health harms. Solvents and open flames demand careful distance, and working in a well-ventilated fume hood helps keep vapors away from skin and eyes. Inhalation can cause irritation, and spills need attention right away because the odor lingers and signals potential exposure. Safety glasses, gloves, and splash protection are not optional in most professional settings. Keeping updated with GHS-compliant labels, spill plans, and proper venting isn’t just red tape—it’s the reason why accidents rarely become disasters. Over time, regular safety refresher training pays off, especially for teams with new members.
This compound has found a home in more industries than one might guess. In flavors and fragrances, 2-Acetylthiophene provides the earthy undertones for things like baked goods or caramel-type aromas in tobacco. Pharmaceutical chemists value it as a flexible building block, feeding into pathways that form everything from anti-inflammatory agents to anticonvulsants. Material scientists even use it as a starting node for synthesizing conductive polymers and advanced organic molecules. Its sulfur component gives it unique electronic features that show up in both sensors and solar materials. From my experience, the reach of this molecule grows as people in different fields trade notes and experiment beyond the well-trodden paths.
Research with 2-Acetylthiophene never really stands still. Innovative synthetic chemists look for green alternatives to traditional methods, aiming for less toxic catalysts or renewable starting materials, while analytical teams invent sharper techniques for spotting trace residues. In drug discovery, libraries of thiophene derivatives often begin with 2-acetyl modifications due to their compact structure and reactive flexibility. Advanced R&D now explores photochemical and enzymatic approaches hoping to minimize side products or energy costs, which is critical as industries face rising regulatory and sustainability pressures. Collaborations between academia and industrial partners speed up the pace, especially where unique properties or patentable reactions come into play.
Human and environmental safety remains a major topic, as the volatile nature and sulfur content of 2-Acetylthiophene raise flags with regulators and occupational health experts. Animal studies suggest short-term exposure can irritate mucous membranes, while long-term effects aren’t fully mapped but warrant caution. Waste control is key since breakdown products from thiophenes can stress water treatment plants or soil microbes. Calls for more comprehensive toxicity profiling and clear labeling reflect the growing need to balance industrial value with public health. People in the lab learn quickly that working clean, documenting incidents, and following disposal rules isn’t just about ticking boxes—it’s about not leaving a mess behind for the next shift or the next generation.
Looking forward, 2-Acetylthiophene probably won’t leave the scene anytime soon. Its role in designing next-generation pharmaceuticals, specialty plastics, and precision agrochemicals is hard to replace. More sustainable manufacturing methods and clever recycling could further cut its environmental footprint. The growth of the electric vehicle and OLED markets might boost interest, since novel thiophene-based materials have unique conducting and optoelectronic properties. In the world of green chemistry, teams keep experimenting with bio-based routes and night-and-day monitoring for safer production. There’s a real sense among people who depend on these chemicals every day that the future belongs to those who don’t just make molecules but rethink how to do it better and safer, every step of the way.
People outside the world of chemistry may never mention 2-Acetylthiophene. Yet, for chemists and manufacturers, this sulfur-containing compound shapes the flavor of everyday life in ways that most folks overlook. Its spicy, nutty aroma means you’ve probably sniffed it in food or perfume without ever knowing its name.
For me, a good cup of coffee stirs memories of chocolate and roasted nuts. The way flavors cling to taste buds isn’t accidental. Food companies often use 2-Acetylthiophene as a flavoring agent. In small doses, it gives a baked, almost caramel-like complexity to things like baked goods, candies, and even alcohol. Studies show this compound can mimic the flavor of fried or roasted foods, an edge for snack manufacturers competing in a tight market. One sniff in a flavor lab and you realize why flavorists call it part of their secret toolkit.
Beyond food, 2-Acetylthiophene lands in the world of fine fragrance. For anyone drawn to earthy or tobacco notes in a cologne, this compound helps shape the undertones. My first encounter with a niche perfume featuring it felt like walking through dried leaves in the fall. Perfumers say it rounds out sweet notes, stopping scents from sliding into “too sugary.” The result recalls real life, not just a laboratory fantasy.
In the pharmaceutical industry, 2-Acetylthiophene doesn’t just stay in the background. Chemists turn to it as a starting material for drugs and other specialty chemicals. Its chemical structure, with both a thiophene ring and a ketone group, acts like puzzle pieces ready to connect. For instance, some antibiotics and anti-inflammatory drugs start with molecules like 2-Acetylthiophene before changing through careful transformations. No miracle drugs get made overnight, but these building blocks help speed up the hard work of research.
No chemical comes without concern. In my lab days, a colleague always reminded us: respect what you work with. Skin or eye contact with 2-Acetylthiophene can cause irritation. Spills need swift cleanup and good ventilation. For manufacturers, keeping worker safety front and center matters as much as getting the chemistry right. More companies now invest in personal protective gear and improved extraction systems. Regulations nudge people toward safer handling, but real change comes from building a “think safe, act safe” attitude on every shift.
Many innovations in food and pharmaceuticals sprang from rethinking old compounds. Green chemistry looks for ways to make chemicals like 2-Acetylthiophene from plant-based feedstocks instead of crude oil. Universities have taken up the challenge, publishing work on cleaner, less energy-hungry production methods. These efforts could cut down environmental impact and open new markets for more sustainable products.
So, while 2-Acetylthiophene might seem tucked away in textbooks or industrial catalogs, it wields a subtle influence across food, fragrance, and medicine. It reminds us how small molecules, often overlooked, can shape experiences as familiar as sharing a meal or spritzing on a favorite scent. Each whiff or taste owes something to quiet chemistry behind the scenes.
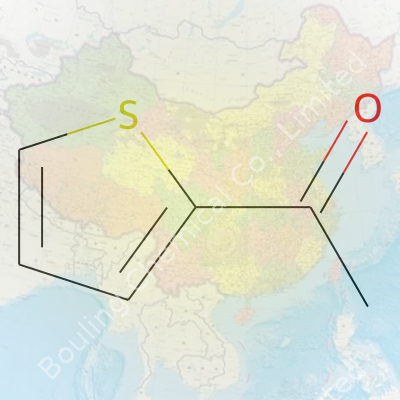
Every molecule tells a story. 2-Acetylthiophene, a name that lands like a puzzle, shows up often in chemistry textbooks and industrial labs alike. Its structure isn’t just some cryptic scientist’s code—this molecule carries both value and function. At its core, the molecular formula spells out C6H6OS. Six carbons, six hydrogens, one oxygen, and a sulfur atom. Feels compact, tidy, and quick to remember.
In labs, accuracy counts. Eyeballing doesn’t cut it. Measuring out 2-Acetylthiophene without knowing its molecular weight would make no sense, like baking without flour measurements. The molar mass clocks in at 126.18 grams per mole. It’s not just a number—it guides how much you use or buy, prevents waste, and allows precise reactions. Years ago, one unexpected miscalculation switched a batch from gold to junk; true for me during a summer internship, where a lazy calculation caused hours of clean-up. Weight matters.
What’s the point of rattling off formulas and weights? 2-Acetylthiophene pops up in scent creation, pharmaceutical research, and academic experiments. Perfume makers use it for earthy, nutty aromas that remind people of roasted bread or coffee. Think of that moment biting into crusty sourdough—the secret aromatic compounds sometimes include this molecule. While many people never see the chemical’s name, they experience its traces.
It also acts as an intermediate, a sort of chemical bridge. Industries use it to build more complicated compounds, including some pharmaceuticals. Organic synthesis relies on stepping stones like 2-Acetylthiophene, not just on flashy big molecules. Back in my undergrad days, chasing a project deadline meant relying on intermediates that just performed, without show or fuss. These behind-the-scenes chemicals keep the system running smoothly, quietly fueling discovery and manufacturing.
Lab mishaps usually don’t make headlines, but behind closed doors, everyone keeps a tight eye on chemicals. Handling something like 2-Acetylthiophene requires more than a good memory for numbers. Gloves, goggles, and proper ventilation save trouble—one careless move with any chemical, even familiar ones, and things go sideways fast. Many researchers start out thinking small molecules don’t pack much punch. One splash or mislabel later, everyone pays more attention.
If there’s a sticking point for those who use or make 2-Acetylthiophene, it’s almost always about handling or supply. Better labeling, double-checked math, and knowing your source help everyone in the pipeline. I remember sharing tips about reliable suppliers and clear labeling. Some labs run on paper-thin budgets, so every gram and every correct label can keep things running and safe. If regulations shift or supply chains tighten, those small habits and networks matter even more.
In chemistry, facts like C6H6OS and 126.18 g/mol act as more than just trivia. They shape real work, keep people safer, and help craft the products and experiences people enjoy every day. For anyone who handles it, understanding these points goes a long way—beyond the formula into real life.
2-Acetylthiophene, with its sharp, nutty scent, lands in the hands of many chemists every year. It pops up in labs working on pharmaceuticals, fragrances, or specialty materials. Some people might not give much thought to storage, but as anyone who spends time around chemicals knows, those little brown bottles on the shelf carry risks and quirks of their own.
Chemicals like 2-Acetylthiophene often make you say “just another solvent,” but one leaky bottle or one forgotten order can create a real mess. The biggest risk with this compound isn’t some Hollywood-style explosion. Instead, you’ll get fumes filling the lab or product breakdown long before the expiration date. Air and moisture help break this stuff down, and sloppy storage speeds up “acetyl” smells turning sour. Once, in a university lab, a careless student left a cap loose—not even overnight—and the smell clung to everything for weeks. Neighbors weren’t fans.
Moisture is the enemy. Even if a label says “stable under normal conditions,” humidity shortens shelf life. Glass amber bottles, often with PTFE-lined caps, block out both light and water. Metal might react and plastics often breathe a bit—glass gives the best chance at purity months down the line. Some research groups keep silica gel right in the storage cabinet. You don’t need a bank vault, but a dry, dark cupboard makes a difference. I’ve pulled bottles from both damp basements and clean, dry storerooms—the stuff from dry, dark spots works every time, no surprises.
Warmth makes chemicals lively, and that’s rarely good news in storage. At room temperature, 2-Acetylthiophene stays pretty calm, but a hot storage closet right next to a steam pipe ruins more than just perfumes. Regular lab temperatures—think 20°C or so— let you hold onto reactivity without springing leaks or making the smell stronger. If your region hits high temperatures, refrigeration helps. Not frozen—just cooler than an attic in July. On summer days, our lab’s chemical fridge puts in real work. After a warm month, stock stored in “cool and dry” is always ready for research.
It’s tempting to think, “I’ll remember what’s in this bottle,” but experience says otherwise. I’ve lost count of unlabeled chemical containers that ended up in the waste bin, each a waste of time and money. Make it a habit: write the name, date received, your initials, and, if possible, purity. If something goes wrong—discoloration, losses, unexplained fumes—you have a trail to follow. It takes less than a minute, and it saves a lot of cleanup.
Even careful workers bump shelves or drop bottles. In one shared lab, I saw a brand-new bottle hit the floor, and the smell followed staff around for hours. Keep spill kits nearby, gloves handy, and a fume hood open if possible. Good storage lowers risk, but a backup plan makes sure a little mistake doesn’t close down the whole building.
Store 2-Acetylthiophene in clean, dry, amber glass in a dark, cool cabinet. Avoid plastic and cheap caps. Label every bottle, track the purchase date. Check for leaks, especially after moving chemicals. It takes a few minutes but saves time—and noses—down the road.
Science labs smell all sorts of ways, but 2-acetylthiophene brings a sharp, earthy scent you won’t forget. Used in flavors, fragrances, and some research, it looks pretty harmless—clear, a touch yellow, moves like oil. It isn’t. Even one whiff too many, some on your skin, or splash in your eye, and you might remember that sting for weeks. The first thing any worker or student should do is read the Safety Data Sheet. That little booklet isn’t just paperwork—real incidents come from skipping the basics.
I learned early that even chemicals with fancy names bite like the rest. Nitrile gloves, chemical goggles, and a proper lab coat mean fewer surprises. Once, someone rolled up their sleeves thinking a dash of solvent wouldn’t matter. That arm burned red for hours. 2-acetylthiophene irritates skin, eyes, and throat, so treat it like you’re handling hot peppers that love to sneak up your nose.
No open-toed shoes around this stuff, either. I once saw a glass vial tip over, shatter, and splatter on a coworker's foot. They got lucky—only a scare. In my view, labs often forget about feet, but a spill will find the smallest weakness.
Hoods cost money, but they do the heavy lifting in keeping noses happy and lungs clear. Pouring or mixing 2-acetylthiophene on an open bench? That’s inviting fumes to linger. Those vapors irritate the airways and can give headaches. Some folks get a rash just by being in the same room for long enough. Tuck projects under a fume hood, and save yourself trouble. Airflow matters—regular rooms don’t cut it for handling volatile compounds.
Distraction leads to mistakes. I’ve seen chemicals put in old water bottles or flasks without a proper label, and that’s trouble waiting to happen. 2-acetylthiophene doesn’t come with a colored warning, so all it takes is a mix-up for someone to get a dose they weren’t expecting. Always label every container, double check caps, and lock up bottles at the end of the day.
Don’t pipette by mouth, ever. That sounds obvious until deadlines roll in and people start taking shortcuts. Spills get cleaned up with absorbent material, straight into chemical waste—not the trash. Bad habits add up.
Accidents happen, even with the best habits. A spill kit for 2-acetylthiophene needs gloves, goggles, and enough absorbent to soak up a real mess. Soak, scoop, and bag it for hazardous waste disposal. No dumping down the drain. I've watched even seasoned chemists panic during a spill. Fast response comes from clear practice, not just reading about it.
Some folks shrug about chemical waste, but one slip-up with the wrong disposal and the whole lab shuts down. 2-acetylthiophene waste goes into a clearly marked bottle—nothing else. Don’t mix wastes unless you’re told. Improper storage can start fires, ruin drains, or tear up equipment. Regular pick-up schedules keep labs from getting crowded with danger.
So many lab injuries come from rushing or not paying attention. Respect for the dangers of 2-acetylthiophene creates safer spaces, whether you’re doing research, flavor chemistry, or quality control. Good habits turn safety from a chore into just another step. Supervisor walkthroughs, updated training, and ready-to-go safety gear all matter, not just to follow rules but to make sure folks want to come back to work tomorrow.
2-Acetylthiophene doesn’t often show up in mainstream conversation, but in labs and factories, its name pops up regularly. Folks who work in the chemical industry know it well—it’s a building block for other chemicals and flavors, and sometimes even pharmaceuticals. So nobody just grabs the first bottle they see; purity becomes a real concern.
Plenty of chemicals show up with a string of nines in their label—like 98%, 99%, or even 99.9% pure. 2-Acetylthiophene is no exception. The reason for all these numbers is more than marketing. Tiny changes in purity can flip a process on its head. I’ve seen a research project fall apart because the solvent had an extra half-percent water content. If that happens with a sensitive catalyst or detector interacting with a batch of 2-Acetylthiophene, the whole experiment could give false results. That’s months of work down the drain.
If a chemist in a food flavor company buys 2-Acetylthiophene, they make sure to check the food-grade certification. A trace of an unwanted impurity can spoil not just the flavor, but even safety certificates. Pharmaceutical labs don’t cut corners either. Imagine a drug contaminated by a few extra molecules of a leftover solvent—that spells huge risk and lawsuits. So, suppliers offer pharmaceutical, food, and industrial grades. Each has its own paperwork and quality checks.
Higher purity comes at a cost. Sometimes a factory using 2-Acetylthiophene to dye plastics doesn’t need the purest batch. It just has to not spoil the end product’s color or texture. But if it’s going into human bodies, like in medicine or flavoring, there’s no room for sloppiness, and prices rise fast for that peace of mind.
Most chemical suppliers know their market. I remember talking to a chemical sales manager who walked me through their catalog: technical grade for industrial valves, reagent grade for education and lab work, ultra-pure for electronics or medicine, and so forth. They run purity tests, sometimes give a certificate of analysis, and provide a safety sheet. The best ones even let buyers see results from their last purity check.
Contamination sneaks in during packing, shipping, or even storage. Labs and manufacturers have stories about “strange smells” in a delivery—sure sign something’s wrong. Testing every new lot saves a lot of heartache, but adds costs. Some buyers, especially in smaller labs or in cost-strapped industries, roll the dice with lower grades. Mistakes happen, and it’s usually the end user who deals with the fallout.
Tougher standards only help if folks actually pay attention and don’t just sign off on paperwork. I always tell new lab workers to double-check every certificate and to run a test of their own. It seems simple, but mistakes almost always come from overlooked basics.
Suppliers can also work more closely with clients, sharing testing data and even helping with troubleshooting. It’s better for everyone—fewer recalls, happier customers, and less waste. As chemical supply chains tighten up, those doing the right thing with purity will hold their ground while the rest scramble.
| Names | |
| Preferred IUPAC name | 1-(Thiophen-2-yl)ethan-1-one |
| Other names |
2-Acetyl-1-thiophene Thiophene-2-yl methyl ketone 2-Thienyl methyl ketone |
| Pronunciation | /tuː əˌsiːtɪl θaɪˈoʊfiːn/ |
| Identifiers | |
| CAS Number | 88-15-3 |
| Beilstein Reference | 1209246 |
| ChEBI | CHEBI:141455 |
| ChEMBL | CHEMBL263170 |
| ChemSpider | 7197 |
| DrugBank | DB01830 |
| ECHA InfoCard | 03d382e8-cf34-42f2-9846-c4d1c5a4bde7 |
| EC Number | 211-934-1 |
| Gmelin Reference | 6737 |
| KEGG | C02354 |
| MeSH | D000197 |
| PubChem CID | 7005 |
| RTECS number | AB1925000 |
| UNII | Q364N215N5 |
| UN number | UN1989 |
| Properties | |
| Chemical formula | C6H6OS |
| Molar mass | 154.22 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | sweet pungent odor |
| Density | 1.13 g/mL at 25 °C (lit.) |
| Solubility in water | slightly soluble |
| log P | 1.64 |
| Vapor pressure | 0.58 mmHg (25°C) |
| Acidity (pKa) | 7.2 |
| Basicity (pKb) | pKb: 7.89 |
| Magnetic susceptibility (χ) | -58.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.536 |
| Viscosity | 1.267 cP (20°C) |
| Dipole moment | 1.96 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 167.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -33.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2532 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | '' |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | Hazard statements: "H302, H315, H319, H335 |
| Precautionary statements | Precautionary statements: P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | Flash point: 84°C |
| Autoignition temperature | 571°C |
| Explosive limits | Explosive limits: 1.2–7.5% |
| Lethal dose or concentration | LD50 oral rat 820 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 3000 mg/kg |
| NIOSH | KH5950000 |
| REL (Recommended) | 500 mg |
| Related compounds | |
| Related compounds |
Thiophene 2-Acetylfuran 2-Methylthiophene 3-Acetylthiophene 2-Acetylpyridine |