Chemists first recognized thiophenes in the mid-1800s, shuffling through coal tar and discovering the distinct sulfurous aroma that set them apart. As the world pushed deeper into organic chemistry, especially in the early 1900s, 2-acetyl thiophene emerged as a compound of interest. The need to better understand heterocyclic chemistry pushed forward many sulfur-containing compounds, but 2-acetyl thiophene stood out due to its acetyl group, offering both reactivity and a handle for further modifications. My own experience with academic research groups has shown that every generation of chemists builds on what came before, and 2-acetyl thiophene's development is no exception; it echoes the dogged pursuit of molecular control and the search for practical syntheses that can stand the test of time and scale up in industrial settings.
2-Acetyl thiophene, a sulfur-containing organic molecule, brings a unique blend of fragrance and reactivity that’s hard to ignore in both research and manufacturing. It often pops up as a pale yellow liquid, carrying a strong nutty odor with a hint of bread crust, likely why food chemists and perfumers continue to eye it as a flavoring and fragrance compound. My time in the lab taught me to respect its balance—a molecule stable enough for storage and handling, yet reactive enough for transformations. Industrial producers package it to precise specifications for pharmaceuticals, flavors, and agricultural chemicals, supporting both product consistency and regulatory compliance.
2-Acetyl thiophene carries a molecular formula of C6H6OS and a molar mass of around 126.18 g/mol. The boiling point generally rests between 190–195°C, while the melting point tends to fall far below room temperature, keeping it a liquid under most lab conditions. Its density sits near 1.13 g/cm³, making it denser than water. I’ve found its solubility somewhat limited in water, but quite good in typical organic solvents like ethanol, ether, and chloroform. Strong odors drift from even small spills, an everyday reminder of its volatility. Chemists value its electrophilic carbonyl group and the electron-rich thiophene ring, which open doors for both nucleophilic attacks and substitution reactions. These features give it the edge in synthetic projects.
Suppliers label 2-acetyl thiophene with its Chemical Abstracts Service (CAS) number 88-15-3, often adding batch numbers, purity levels (usually 98% or higher for lab or pharma grade), and hazard designations. Containers must bear the flammable liquid icon, and shipping follows the protocols laid out by international transport authorities. In my experience, missing or unclear labeling slows everything down and complicates audit trails. Safety data sheets need to list exposure limits, first aid recommendations, and disposal requirements, helping everyone from lab techs to supply chain managers handle it right on each step of the journey. Analytical specifications confirm its identity by GC or HPLC while limiting impurities such as sulfur dioxide, moisture, or chlorinated byproducts.
Industries turn to Friedel–Crafts acylation routes, often reacting thiophene with acetic anhydride or acetyl chloride in the presence of aluminum chloride as a catalyst. This approach leverages the natural reactivity of the thiophene nucleus while favoring substitution at the 2-position thanks to electron distribution. The process kicks out hydrogen chloride as a byproduct, demanding careful venting and scrubbing steps. My own work with small-scale synthesis has shown that controlling moisture and temperature during this procedure is crucial to achieving high yields and stopping side reactions. On a larger scale, innovations focus on greener solvents and catalytic systems that reduce waste and energy consumption.
2-Acetyl thiophene’s molecular core invites a range of chemical manipulations. In the hands of a creative chemist, reductions with sodium borohydride can convert the acetyl group to a secondary alcohol. Halogenation at the ring opens doors for subsequent coupling steps in medicinal chemistry. Oxidation provides access to corresponding acids or more oxidized sulfur-containing frameworks. Day in and day out, labs use this molecule in building blocks for heterocyclic compounds, agricultural agents, and advanced materials. Its reactivity enables the creation of dyes, active pharmaceutical ingredients, and sometimes electronics intermediates. Each chemical tweak banks on the robust stability of the thiophene ring, with selectivity driven by both electronics and steric effects.
2-Acetyl thiophene won’t always appear under that name on a bottle. Other monikers include Thiophene-2-yl methyl ketone, 2-Thienyl methyl ketone, and Acetylthiophene. Catalogs sometimes shorten it to 2-AT. These aliases reflect both its place in the realm of ketones and its heritage as a thiophene derivative. Older literature and patents may use local naming standards or proprietary codes, which can confuse sourcing and quality assurance teams. Through continual cross-checking, chemists and procurement specialists prevent mix-ups that could otherwise derail experiments or production.
Dealing with 2-acetyl thiophene, you respect its flammability first and foremost. The liquid evaporates quickly in open air, pushing vapors that catch fire with minimal ignition sources. Direct skin contact irritates, so gloves and goggles become non-negotiable. Spilled product leaves a strong, lingering odor, and the vapor can irritate eyes and mucous membranes. Working in well-ventilated fume hoods with explosion-proof equipment became a habit for me, reinforced by mandatory safety training. Regulatory agencies like OSHA and ECHA demand risk assessments and regular monitoring. Waste must be collected according to hazardous chemical guidelines, avoiding any uncontrolled release into drains or the air.
The reach of 2-acetyl thiophene spreads far. Its heavy use in flavor and fragrance industries owes to its nutty, corn-like scent, which pops up in baked goods, tobacco, and beverages. Pharmaceutical firms use it as a synthetic intermediate, building out complex scaffolds for antihistamines, antifungals, and anti-inflammatory drugs. Agrochemical researchers push its derivatives into new pesticides and fungicides, valuing the ring’s stability in harsh field conditions. Specialty chemicals benefit from its presence in dyes and pigment production, as the sulfur atom provides color properties unavailable in purely aromatic compounds. Each of these uses builds on its proven ability to deliver both performance and regulatory compliance in finished products.
Not a year goes by without new scientific papers probing the next breakthrough involving 2-acetyl thiophene. Medicinal chemists have published methods for connecting the thiophene core to biologically active side chains, chasing after new drug candidates. Green chemistry initiatives aim for synthesis using recyclable catalysts and reduced-waste procedures—with some teams developing processes using solid acids or ionic liquids instead of classical Lewis acids. Analytical research improves detection and quantification, deploying advanced mass spectrometry to track trace impurities, which matters especially in pharmaceutical environments. Many of my colleagues argue that the molecule’s future value comes from these steady, practical improvements that make production safer, cleaner, and cheaper for everyone involved.
Early toxicology studies found that 2-acetyl thiophene poses moderate hazards if handled carelessly. Acute exposure through inhalation, ingestion, or skin contact can cause irritation and, at high doses, symptoms like headache or dizziness. Animal studies at exaggerated exposure levels showed effects on the liver, though not significant carcinogenicity or mutagenicity under normal circumstances. Regulatory agencies set occupational exposure limits and mandate clear hazard communication. Ongoing toxicity studies dig deeper, aiming to define safe limits for workers and consumers, especially in industries where the molecule gets added to food or fragrances. From a practical point of view, maintaining up-to-date safety protocols and providing clear training has proven the most effective path around possible risks.
Looking ahead, 2-acetyl thiophene sits at a turning point where sustainability and performance must go hand-in-hand. The rising focus on clean-label ingredients pushes flavor and fragrance manufacturers to justify every molecule in their blends, so making the supply chain more transparent grows more urgent by the year. Regulatory scrutiny of process chemicals nudges innovation toward less hazardous syntheses and improved recyclability. In pharmaceuticals, new routes may favor biocatalysis or flow chemistry, reducing both waste and production time. Digital automation speeds up both quality control and compound tracing, driving tighter control over both the materials and their PR image. The underlying chemistry hasn’t changed, but how people interact with it—and the rules they play by—keep evolving, keeping opportunities open for those able to adapt and improve on what came before.
Most people won’t recognize the name “2-acetyl thiophene,” but plenty have encountered its effect. It’s one of those tiny molecules shaping tastes and smells that spark memories and push industry. Used mostly in flavors and fragrances, 2-acetyl thiophene brings a roasted, nutty, almost popcorn-like aroma that’s tough to replace. Food makers blend it into everything from baked goods to candy, chasing that warm, inviting smell that makes us reach for another cookie.
That scent isn’t only for snacks. Tobacco companies have leaned on 2-acetyl thiophene for decades, blending it into tobacco or vape flavors to smooth out rough edges and boost complexity. It plays well with caramel or chocolate notes, which helps in crafting those trendy e-liquids with layered dessert vibes. Perfume houses and soap makers add it for depth and subtlety, as it works well with both sweet and savory themes in fragrance.
Go past the aroma, and 2-acetyl thiophene finds another job. Chemists depend on it as a useful building block. Its thiophene ring with an acetyl side group makes it attractive for synthesizing new pharmaceuticals and specialty chemicals. Researchers use it to develop anti-inflammatory agents, antibiotics, and even certain dyes. Without small and flexible molecules like this, modern medicinal chemistry would run into walls that block progress.
2-Acetyl thiophene rarely makes headlines, but safety isn’t an afterthought. The FDA and food safety agencies in Europe allow it in food at low levels, due to its manageable toxicology profile. Long-term worker exposure or heavy doses could pose risks, so factories set guidelines for air handling and personal protection. Workers in chemical plants tell stories about the “corn chips lab day” when the air gets thick with synthetic aroma. Responsible operations, good ventilation, and clear labeling keep risk in check.
The compound usually comes from synthetic routes, since extracting it from natural sources doesn’t make sense cost-wise. Most of it results from reacting thiophene with acetic anhydride using acid catalysts. These routes work at industrial scale, but not all chemical suppliers control emissions or manage waste equally. Certain organizations push for greener chemistry, calling for less hazardous solvents or lower energy use. As regulations tighten and consumer pressure grows, demand for environmental transparency rises. Producers that provide clear data about emissions and sustainable sourcing tend to earn better trust from big brands and international buyers.
In my own time working with food companies, traceability and origin have become big topics. Chefs and small producers want to know where every flavor molecule comes from, especially when it goes into “clean label” or natural-branded foods. That scrutiny shines a light on supply chain weaknesses. Sometimes smaller players buy from brokers with little oversight, which leads to inconsistent quality or even contamination. Government agencies and advocacy groups regularly test imported flavor chemicals for purity and fake additives. Fines and news stories push the industry to step up. Adding blockchain or digital tracking tools could help build a better record, so ingredients like 2-acetyl thiophene earn trust beyond the factory gate.
People might not realize the care, chemistry, and regulation driving the flavors behind favorite foods or fragrances, yet molecules like 2-acetyl thiophene carry real weight. Being transparent, reviewing safety, and investing in greener synthesis keeps this unsung hero working safely—and keeps those sweet, toasty moments coming.
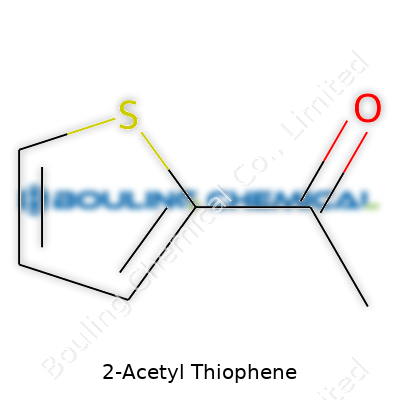
The formula behind 2-Acetyl Thiophene looks straightforward: C6H6OS. Six carbons, six hydrogens, one oxygen, and one sulfur. The molecular structure frames it up as a thiophene ring, much like benzene but swapping a carbon for sulfur, and a key acetyl group attached at the second position. Those working in labs spend a lot of effort getting these details right, since one wrong tweak at the atomic level can turn a promising drug candidate into frustration.
I spent years in an academic lab, and there’s always a hundred bottles lined up on the shelves, labeling faded. One, marked “2-Acetyl Thiophene,” didn’t last long. Chemists turn to it often, relying on its unique scent and chemical versatility. Its aroma often lands in flavor and fragrance industries, hinting at bread or roasted nuts. Whether blending perfumes or teasing out natural aromas in cocoa, this compound delivers more than the sum of its parts.
A scientist quickly sees potential wheels turning: its structure means it bridges the worlds of organics and heterocyclic chemistry, where oxygen and sulfur atoms play differently around carbon backbones. The molecular formula lays the groundwork for recognizing reaction patterns and safety protocols. Without the right formula, data sheets lose meaning and a small error turns into safety hazards and wasted resources.
I remember cataloging chemicals and double-checking their formulas, cross-referencing hand-written notes and digital databases. Molecules like C6H6OS require this precision. Mistakes here don’t just impact the lab; they ripple out to supply chains, product labeling, and even safety data sheets that protect workers on the manufacturing floor.
Added vigilance in the formula not only prevents costly missteps, but also meets regulatory demands. In Europe, REACH compliance requires full transparency down to the molecular level, so listing the correct structure isn’t about paperwork—it protects public trust and business reputation. In the United States, EPA filings mean every detail becomes public record, making accuracy essential rather than optional.
The real innovation comes from those who not only see a molecular formula as a string of letters and numbers, but as a starting point for smarter synthesis and sustainable choices. 2-Acetyl Thiophene ends up used in research on new pharmaceuticals and agrochemicals due to its reactive sites. Authorities and industry both benefit if the properties and environmental impacts are communicated with full accuracy, matching the actual molecule present.
Training next-generation chemists to respect not just the formula but also the context pays off. They learn quickly that attention to C6H6OS’s details can make or break experiments. Several companies are leaning toward digital inventory systems that cross-check formulas against verified sources and flag inconsistencies. These steps help reduce errors and make traceability easier when questions come up later.
At its root, the molecular formula of 2-Acetyl Thiophene—C6H6OS—serves as a silent contract. Respecting the reality behind the formula means better safety, stronger science, and products that do what they claim, from fragrances to pharmaceuticals. Focusing on chemical literacy, quality control, and openness around data encourages everyone to create and use compounds safely and responsibly, while keeping curiosity alive.
2-Acetyl thiophene brings some unique challenges into the workspace. Anyone who has handled this yellowish, aromatic liquid remembers its strong smell and the questions it raises about safety. Thiophene compounds have found a place in pharmaceutical and fragrance labs, but their use calls for careful respect. The liquid can irritate skin, eyes, and the respiratory tract. Accidental splashes sting, while vapor can tickle your nose in the wrong way. More than once, I caught a whiff while moving a bottle between hoods and felt an immediate reminder: fume protection isn’t just for show.
A long lab coat keeps your sleeves and regular clothes clean, but gloves pull the real weight. Nitrile gloves have kept my hands safe from spills, though double-gloving makes sense if you plan to handle larger volumes. Splash goggles beat regular glasses every time; splashes bounce off curved plastic instead of clothes or eyelids. Working without eye protection courts trouble, especially with volatile organics.
Don’t underestimate the need for ventilation. Even what looks like a simple transfer should take place inside a chemical fume hood. Good airflow draws away the vapors before they reach your face. I’ve watched fumes drift in still air, especially on warm afternoons—and taking a deep breath isn’t worth the risk.
Mistakes still happen, no matter how careful you feel. Once, a junior held a bottle loosely and knocked it on the benchtop. The minute pool that formed looked small, but the smell hung for ages. Spill kits stocked with absorbent pads and neutralizing powders make cleanup less frantic. We always tackled spills with gloves and goggles, then carefully disposed of the soaked materials as hazardous waste. The regular trash is off-limits with anything that can burn or react unpredictably.
Storage can prompt its own set of headaches. Flammable liquids cabinet—metal, with an obvious warning sticker—is where all of our thiophene compounds lived. Even on a crowded shelf, the bottles never mingled with acids or oxidizers that might trigger a reaction. Room temperature and a dark spot help keep the liquid stable. Labeling remains crucial. More than once, a chemist borrowed from the wrong bottle by mistake, so dates, names, and warning symbols became non-negotiable.
Safety gear feels like a hassle until something goes wrong. I once saw a new technician reach for a chemical eye wash after a splash—she had been through the training last month, so panic didn’t kick in. Knowing where to find showers, eyewash fountains, and fire extinguishers cuts down on response time. Regular drills and reminders go a long way. No one wants to fumble during an emergency.
Following safety rules feels easier with the right workplace attitude. Supervisors who wear their own PPE every day send the message that everyone’s safety matters. Posting up-to-date safety sheets near storage areas gives quick access when people forget the fine print about health hazards or recommended procedures. I’ve seen workplaces where staff shared stories about close calls, turning them into lessons for the whole team instead of covering up mistakes. In labs that encourage questions, less experienced colleagues pick up safe habits quickly.
Anyone who’s ever worked in a laboratory or dabbled with syntheses knows the boiling point isn’t just a line in a handbook. In the case of 2-acetyl thiophene, that value—listed as roughly 227°C—can steer everything from safety protocols to the success of a reaction. I remember my early days in organic labs, sweating over distillation rigs, watching compounds creep up the neck of a flask. More than once, underestimating boiling points landed a project in the “start over” pile. With 2-acetyl thiophene, misjudging its volatility can bring headaches no one wants.
Boiling point comes from molecular structure—no mystery there. 2-acetyl thiophene bears a sulfur atom in its five-membered ring and a carbonyl group. This structure leads to a heavier, slightly polar molecule. The sulfur and oxygen create a higher boiling point than simpler ethers or hydrocarbons of similar mass. I’ve seen people assume it behaves like other acetyl compounds or thiophenes, only to run into problems during purification. That extra heat requirement can catch a technician off guard, especially if the equipment isn’t cut out for sustained high temperatures.
2-acetyl thiophene pops up in flavor chemistry, pharmaceutical intermediates, and even as a base for more complex molecules. Lab-scale experiments rarely impact many outside the facility, but industrial-scale production, where pounds or tons are heated, changes the conversation. Hit that boiling point, and you face risk of inhalation exposure or even fire if vapor control systems aren’t up to the task. In my circle, best practice always meant double-checking those values before scaling up heating methods. Overconfidence led to pressure spikes and plenty of sleepless nights for more than one chemist.
Sources like the Merck Index or Sigma-Aldrich place 2-acetyl thiophene’s boiling point in a tight 227–229°C range. This might sound dry, but the consistency speaks to reliable data collection. Ignoring those numbers or assuming minor deviations can lead to product loss, equipment failure, or personal injury. International safety data sheets recommend high-temperature gloves and proper fume hoods for a reason—trace amounts of vapor at those temperatures shouldn’t be taken lightly. Even the smell of thiophenes can tell you the air’s become a little too saturated with organic vapor. Memories of that sharp, sulfur tang only reinforce the need for respect here.
Solving problems tied to this boiling point isn’t theoretical. Good airflow, temperature monitoring, and clear safety training put disasters in check. I’ve watched teams waste hours troubleshooting residue just because a distillation flask wasn’t rated for prolonged high heat, so equipment checks go hand-in-hand with chemical knowledge. Digital temperature probes, robust chillers, and scrubbers for vapor management transform what could be a sketchy process into a routine lab day.
Skipping over technical details can wreck research or cost money on the production floor. Data transparency and taking the time to understand each chemical—especially something like 2-acetyl thiophene—builds a safer and more productive work culture. For anyone moving past textbooks and into the lab, the lesson sticks: measure twice, heat once, and know your numbers before lighting the burner. A boiling point isn’t just trivia; it’s a checkpoint on the way to solid science.
2-Acetyl thiophene isn’t the sort of chemical that just sits harmlessly on a shelf. This compound, with its nutty, bready aroma, finds use in flavors, fragrances, and sometimes in labs developing new molecules. People who’ve handled it know the liquid packs a distinct punch. The reality is plain: it can irritate the skin and eyes, and if you leave it out without proper care, it’ll degrade or react with air and light. That spells trouble for both safety and the quality of your inventory.
2-Acetyl thiophene is flammable. This alone should get attention. In a real-world setting, even a minor spill becomes a hazard if it meets sparks or a hot surface. The fumes aren’t just strong; breathing them for too long irritates your sinuses and even your lungs. Every worker in a lab or warehouse knows stories about chemicals in rusty cans or old bottles. Old containers leak vapor, and irritating smells alert you only after things have started going wrong.
It’s tempting to stash flavor chemicals next to paints, solvents, or cleaners in a shared closet. But mixing storage environments leads to cross-contamination and unexpected reactions. Once, I saw a small warehouse go through a costly inventory loss just because incompatible chemicals sat together a few months too long, and the degradation didn’t show until someone opened a container. Segregating chemicals based on their behavior — acidity, flammability, and toxicity — isn’t overkill. It prevents real accidents.
Keeping 2-acetyl thiophene in a cool, dry, and well-ventilated room makes sense. Heat speeds up breakdown and can even build up pressure in closed bottles. The storage container ought to be tightly sealed, made of glass or chemical-resistant plastic. Cheap caps erode; I’ve seen them crumble when someone tries to close a container too tightly or leaves it open for cleaning.
Darkness gives added protection. Sunlight or strong indoor lighting speeds up chemical changes, so don’t just rely on the opaque label or box. People use brown glass bottles in labs for a reason. A locked, labeled cabinet shields it from accidental access — it keeps the curious or the unwary from picking up the wrong bottle, and signals that this isn’t just kitchen flavoring.
Simple steps save lives. Keep a spill kit — not just mop-up pads, but proper neutralizers and protective gloves. An eyewash station shouldn’t be a distant luxury. If fumes escape, you want an open window or fume hood nearby, not a scramble across a crowded stockroom. Labels telling you the exact content, date of storage, and hazard symbols beat memory and sticky notes every time.
Workplace culture makes the difference between routine and disaster. Regular training and a storage checklist pay for themselves. When new staff help unpack chemicals, they tend to ask, “Why separate them?” After a few stories about minor mishaps, people keep labels sharp and containers clean. If a facility upgrades shelves or replaces old boxes, the opportunity for new storage systems shouldn’t be missed — fresh shelves that ventilate better, or locking cabinets near extraction fans, go a long way toward safety without weighing down daily work.
| Names | |
| Preferred IUPAC name | 1-(Thiophen-2-yl)ethan-1-one |
| Pronunciation | /tuː əˈsiːtɪl θaɪˈoʊfiːn/ |
| Identifiers | |
| CAS Number | 88-15-3 |
| Beilstein Reference | 1209243 |
| ChEBI | CHEBI:141528 |
| ChEMBL | CHEMBL15411 |
| ChemSpider | 6480 |
| DrugBank | DB04256 |
| ECHA InfoCard | DTXSID8020087 |
| EC Number | 211-047-3 |
| Gmelin Reference | 80918 |
| KEGG | C05878 |
| MeSH | D000196 |
| PubChem CID | 7002 |
| RTECS number | XN6475000 |
| UNII | 410LTG44G3 |
| UN number | UN number: "UN 2810 |
| CompTox Dashboard (EPA) | DJ3200000 |
| Properties | |
| Chemical formula | C6H6OS |
| Molar mass | 140.19 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | sweet, ethereal, penetrating |
| Density | 1.129 g/mL at 25 °C |
| Solubility in water | Slightly soluble |
| log P | 1.49 |
| Vapor pressure | 0.46 mmHg (at 25°C) |
| Acidity (pKa) | 7.2 |
| Basicity (pKb) | pKb = 9.85 |
| Magnetic susceptibility (χ) | -62.6×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.536 |
| Viscosity | 1.110 mPa·s (25 °C) |
| Dipole moment | 2.75 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 256.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -44.3 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2428.8 kJ/mol |
| Pharmacology | |
| ATC code | |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H226, H315, H319, H335 |
| Precautionary statements | Precautionary statements of 2-Acetyl Thiophene are: "P210, P261, P280, P305+P351+P338, P404+P233, P501" Let me know if you need their detailed descriptions. |
| NFPA 704 (fire diamond) | 2-1-0-X |
| Flash point | 54 °C |
| Autoignition temperature | 250°C |
| Explosive limits | Explosive limits: 1.6–9.7% |
| Lethal dose or concentration | LD50 (oral, rat): 1630 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 1530 mg/kg |
| NIOSH | KL3675000 |
| REL (Recommended) | 21°C |
| Related compounds | |
| Related compounds |
2-Acetylfuran 2-Acetylpyridine 2-Acetylpyrrole Thiophene 3-Acetylthiophene |