The story of 2-Acetyl Thiazole stretches back to the golden era of organic chemistry. Researchers sniffed out its distinctive aroma early in flavor science, recognizing something unique about its nutty, popcorn-like scent—something you can catch a whiff of in cooked grains and roasted coffee. In 1936, German chemists published early syntheses, but the compound didn't really break into the mainstream until food research blossomed after World War II. Flavor chemists and perfumers soon realized its magic: a small molecule responsible for so much character in roasted and baked goods. The timeline of its adoption reflects shifting consumer tastes and growing understanding of how small amounts of certain molecules transform how we experience flavor and aroma.
2-Acetyl Thiazole has become a foundation for the flavor and fragrance industry. Manufacturers offer it as a clear to pale yellow liquid, packed in both small bottles and steel drums, depending on whether it’s headed for product development labs or full-scale manufacturing floors. Its worldwide availability can be traced to its versatility; today, producers market it to food, beverage, tobacco, and fragrance companies. Often labeled as nature-identical, the product plays a crucial role in supplying consistent flavor across continents where agricultural variation might otherwise create inconsistency batch by batch.
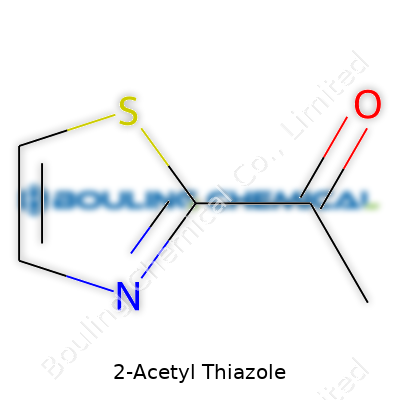
2-Acetyl Thiazole’s striking aroma comes from its molecular structure—C5H5NOS. The compound melts at about 28°C, turning into a liquid just below room temperature, so chemists often encounter it in both solid and liquid states. It boils at 185°C. The density sits near 1.16 g/cm³. In water, it shows limited solubility, but it dissolves easily in ethanol, ether, and typical organic solvents. Those features allow it to blend seamlessly into food, beverage, and perfume formulas. Its structure features a thiazole ring—a nitrogen, a sulfur, and a double bond that provides chemical reactivity and a unique physiological effect on human senses.
Producers stamp every batch with specifications for purity, typically not less than 98%. Inspectors check for color and odor: a clear to yellow liquid carrying a nutty, popcorn aroma. Labels disclose storage requirements—a cool, dry location in tightly closed containers—along with relevant hazard pictograms since the compound requires careful handling to protect skin and eyes. Certificates of analysis document impurity levels and guarantee compliance with strict international flavor safety regulations. Lot numbers trace the history of every drum, supporting food safety recalls and product traceability.
Most batches come from a reaction between 2-bromoacetophenone and thioacetamide, a route that gives good yields and clean products. Laboratories load the reactants into glassware, run the reaction at carefully controlled temperatures, and finish by distilling the product for purity. Some smaller producers stick with an older method: using acetic anhydride and thiazole in a solvent, a process that takes longer and tends to give lower yields. Scaling up to factory output, companies rely on stainless steel reactors and robust filtration systems, making sure the process stays isolated from air to prevent unwanted reactions that create off-flavors or dangerous byproducts.
Chemists often tweak 2-Acetyl Thiazole to change how it behaves in food and fragrance applications. The acetyl group can react with amines, extending chains or adding different functionalities for novel aroma compounds. Nitration or halogenation on the thiazole ring creates derivatives with altered volatility or odor notes, allowing flavorists to tailor products for different foods. Its reactive functional groups also provide an entry for creating more complex molecules—one more reason why research chemists continue exploring what new frontiers lie one step beyond basic 2-Acetyl Thiazole.
Chemists, food scientists, and manufacturers each refer to 2-Acetyl Thiazole in their own ways. You might spot it labeled as 1-(Thiazol-2-yl)ethan-1-one, 2-Thiazolylethyl ketone, or even 2-Acetylthiazole with slight variations in spacing and hyphens. In flavor catalogues, it appears under codes linked to the Food Chemicals Codex or FEMA numbers. Some brands attach unique trade names for proprietary blends. Clear labeling ensures procurement teams and regulatory inspectors know exactly what substance sits inside the drum.
In production and application, safety never becomes an afterthought. Skin or eye contact with 2-Acetyl Thiazole can irritate, demanding gloves, goggles, and well-ventilated workspaces. Producers abide by OSHA rules and international GHS labeling, marking containers with pictograms and safety phrases. In blending facilities, localized exhaust systems draw away any vapor. Teams train for prompt cleanup in case of small spills, using absorbent materials plus neutralizing solutions that prevent environmental release. Storage guidelines state cool, shaded areas—no direct sunlight, no ignition sources nearby. These steps keep employees and the environment safe while allowing the powerful capabilities of this small molecule to shine.
Most people experience 2-Acetyl Thiazole not in laboratories but in the foods they eat. It plays a starring role in snacks—especially crackers, popcorn, and baked treats—where it creates a signature roasted note difficult to achieve with natural ingredients alone. Beverage makers turn to it for nutty undertones in coffee and whiskey analogs. Some tobacco blends contain trace amounts to round out complexity and aroma. Perfumers leverage its richness for warm, gourmand notes in fragrances. Outside of direct food and fragrance use, 2-Acetyl Thiazole helps as a flavor reference in analytical standards, training technicians to calibrate their instruments to the real stuff.
Development teams pressure test formulations with 2-Acetyl Thiazole, pushing for authentic flavor profiles that survive harsh processes like ultra-high temperature food sterilization or long shelf life. Ongoing R&D looks for even better synthesis techniques—safer, greener, and more cost-effective—which cut down on waste and environmental impact. Analytical labs focus on precisely detecting traces of this compound in foods to verify labeling and compliance. Multidisciplinary research blends food science and neuroscience, asking how the compound interacts with taste and smell receptors to influence craving, satiety, and emotional response.
Any substance added to food faces tough scrutiny. Toxicology studies show that at flavor-use levels, 2-Acetyl Thiazole does not cause harm. The FDA and EFSA recognize it as generally safe within strict limits, based on both short-term and lifelong exposure studies in animals and cell cultures. Research tracks breakdown products in the body and environment to ensure no hidden dangers lurk. Some studies suggest high doses may present risks, but food use never approaches such levels. Regulatory bodies require new toxicological data if applications change or exposure increases, keeping public health at the top of the agenda.
Looking ahead, 2-Acetyl Thiazole’s future ties closely to trends in plant-based and clean-label foods. Developers strive to deliver authentic roasted notes to meat alternatives without animal products. The pressure grows for non-GMO sourcing and sustainable manufacturing, nudging chemists toward bio-based production from fermentation or enzymatic routes instead of petrochemical pathways. As sensory science digs even deeper into the links between aroma, memory, and emotion, researchers work to enhance the experience of eating through subtle tweaks to molecules like this one. Industry and academia will keep scanning for any newly discovered health or safety signals, aiming to balance consumer excitement with up-to-date science.
Most folks haven’t heard of 2-acetyl thiazole, but you’ve probably tasted it more times than you can count. This molecule shows up in a lot of foods as a flavor booster. It brings out the earthy, toasted notes in baked bread, roasted coffee, and even some cheeses. Think about that first sip of hot chocolate or biting into a warm croissant—the slightly nutty, almost roasted aroma often owes something to this compound. Food technologists rely on it to create a fuller, richer taste in snacks, sauces, and ready-made meals.
Some additives in processed foods have a bad reputation, but 2-acetyl thiazole offers more than just flavor. Its safety record stands up to scrutiny. Regulatory agencies, including the FDA and EFSA, have assessed and approved it for use in food. My time in food research taught me to look closely at what goes into everyday recipes. Our team kept track of scientific studies on flavor compounds, and 2-acetyl thiazole consistently appeared as a reliable option because it passes safety reviews and doesn't build up harmful residues in the body.
This molecule forms naturally when foods brown or toast. You heat bread in the oven, and proteins and sugars react to produce hundreds of new compounds. Among these, 2-acetyl thiazole stands out for its powerful aroma. Scientists often describe its scent as popcorn-like or reminiscent of nuts. The power of this compound lets food companies use just a small amount to create a real impact, which means lower overall chemical usage.
Perfume makers recognize its value, too. Many fragrances contain coffee or hazelnut notes, and this is where 2-acetyl thiazole shines. I’ve spent afternoons with scent formulators who experiment with the tiniest amounts to get that subtle, warm depth in perfumes or candles. Its cost is modest compared to some rare natural extracts, so it helps keep products affordable without sacrificing complexity.
Nothing is perfect in food chemistry. With flavor compounds, the main risks come from overuse or impurities in raw materials. Ethical sourcing and thorough quality checks must stay at the top of every manufacturer’s list. Reliable suppliers provide high-purity batches, accompanied by lab analysis to make sure nothing dangerous sneaks in. The push for more sustainable, plant-based sources is gaining momentum, too. Biotechnologists are working on fermentation and greener synthesis methods, which could cut down reliance on petrochemicals and lower environmental impact.
The food industry constantly seeks ingredients that can replace salt, sugar, or fat. 2-acetyl thiazole acts as a flavor enhancer, boosting satisfaction in better-for-you options. Training chefs and product developers in natural flavor chemistry means they can make foods both tasty and health-conscious. On the consumer side, clear labeling and transparency help build trust. People deserve to know what’s in their foods and fragrances, and how those choices affect both health and environment.
Understanding why 2-acetyl thiazole appears in foods and scents helps shed light on bigger trends in flavor science and responsible production. The story behind these small molecules reflects the work and diligence of food makers, scientists, and regulators, all aiming to balance pleasure, safety, and sustainability in what we eat and smell every day.
Walk through a bakery and that warm, toasty, almost popcorn-like aroma lifting from fresh bread often comes from more than just flour and yeast. Chemists have identified 2-Acetyl Thiazole as a distinct part of what gives a buttery or roasted note to food. Companies use it to enhance flavors in baked snacks, savory seasonings, and even beverages. This compound has made its way into the flavoring toolbox thanks to its effectiveness at small concentrations—most snacks need only a tiny pinch to create that crave-worthy scent and taste.
I always look at safety records before putting anything in my own kitchen. Government agencies such as the US Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA) keep a close eye on food additives. 2-Acetyl Thiazole shows up as “Generally Recognized as Safe” (GRAS), which sounds official but really means experts agree it doesn’t raise a red flag at the intended usage levels. Trusted authorities only hand out this status after combing over studies, toxicology reports, and reviewing data for side effects or long-term risks.
It’s the dose that makes the poison, even for harmless-sounding ingredients. Manufacturers typically add 2-Acetyl Thiazole by the milligram—enough to tweak flavor but far below levels linked to harm. Studies tracking both animals and people haven’t found evidence of cancer, liver problems, or allergic responses at the concentrations used in commercial food. EFSA’s reports show that they watch consumers’ overall exposure, checking that the amount in a typical diet doesn't cross thresholds established by science. Surveys and market research confirm that most people only get a fraction of the exposure considered risky, even among those who eat flavor-rich processed foods daily.
Sneaky additives have tarnished public confidence in the past. Any company using 2-Acetyl Thiazole must list it on the label—no loopholes. I’ve spent years reading ingredients, and seeing clear, honest labeling means more than any technical explanation ever could. Most reputable brands build trust on transparency, sharing details on sourcing and food safety certifications. Several leading food-grade flavoring suppliers undergo independent audits to make sure their processes meet consistent quality and purity standards.
Research never stops. Scientists keep an eye on potential risks that could show up in sensitive groups like infants, pregnant women, or folks with metabolic conditions. To date, comprehensive human studies do not point to unique dangers, and countries with strict standards—including Germany, Japan, and Australia—support its use. Of course, no system can promise zero risk for every person. My own approach relies on moderation and keeping a close watch on emerging science.
People looking to reduce exposure to food flavorings can check labels and opt for products marketed as minimally processed. Simple habits—like cooking at home and eating a variety of whole foods—make a real difference. Food manufacturers should keep funding safety studies and updating public guidance as new information emerges. For now, 2-Acetyl Thiazole remains a safe and recognizable part of the modern flavor landscape, with strict oversight and decades of use to support its reputation.
Crack the code on a fresh piece of toasted bread or a warm pile of popcorn and you will stumble on a secret: 2-Acetyl Thiazole, an unsung hero playing in the background. Its presence in the world of aroma often hits before someone learns what actually made the smell so addictive. To my own nose, this compound delivers a nutty, roasted edge that stands up against synthetic and natural flavors alike. Plenty of food scientists agree that it shapes some of the best-loved comfort foods—baked and fried goods included.
Professional flavorists and home cooks both stumble over the same puzzle piece. Why does some bread smell like it could leap out of the oven, even when just slightly reheated? The thiazole ring structure shows up in food chemistry as it forms during Maillard reactions—the same heat-driven process that browns a steak or crisps the top of a casserole. 2-Acetyl Thiazole specifically traces back to reactions between amino acids and sugars, creating a scent that stacks on green, bready, and popcorn-like notes. Its power comes through even at tiny concentrations—sometimes less than a single part per billion reshapes the scent of a room.
Draw in a deep breath and you may pick up a lot more than just “toast.” The classic description combines roasted, savory, and slightly sulfurous elements. Imagine a day-old bread crust, a whiff of corn chips, or the golden foam on a light beer. There’s a sharpness that makes it easier to believe real cheese or a perfect butter might be hiding close by. That’s what made it valuable for snack food makers. Through years of working around commercial kitchens and R&D panels, experts learned to trust their noses for the punchy edge of 2-Acetyl Thiazole to bring out “authentic” fried aromas in products as varied as crackers or even cooked rice.
Rolling out flavors based on science runs into obstacles. This compound works best at very low use rates—too much and the food flips from inviting to overwhelming. Some people experience a harsh, medicinal tang after longer exposure. This puts the onus on how closely food producers can measure and control tiny doses. Without proper training or guessing on ratios, one batch of crackers starts tasting like burnt plastic. We find more brands leaning on sensory panels and gas chromatography to back their choices with data, not just hope.
Benchtop scientists want to know if there’s a downside to heavy synthetic use. Regulatory agencies in the US and Europe give the green light for food use, but questions linger around allergenic potential, long-term exposure, and effects on children. Crafting 2-Acetyl Thiazole from bio-based methods, rather than only fossil routes, takes some burden off the system—and makes companies sleep easier about their environmental impact.
Every time someone sits down with a fresh bag of chips or breaks into a baguette, thousands of chemical signals hit the senses. 2-Acetyl Thiazole joins other natural notes to create something both familiar and hard to pin down. On the consumer side, transparency about what goes into flavoring isn’t just marketing; it keeps eaters with allergies or sensitivities in the loop. As ingredient lists get longer, reminding ourselves what these compounds do—down to their aroma fingerprint—brings the science closer to home.
Most people who have tasted fresh-baked bread, roasted coffee, or ripe tomatoes have enjoyed an aroma that lingers longer than the flavor itself. That magic boost both up close and in the kitchen often comes from chemicals like 2-Acetyl Thiazole. Assigning this molecule a specific number, the CAS number 24295-03-2, lets anyone from a food scientist to a regulator know exactly which compound they’re dealing with, no matter what local nickname or shorthand pops up.
Numbers rarely tell a story at first glance, though in chemistry, they give just enough certainty for work to move forward. CAS numbers, handed out by the Chemical Abstracts Service, serve as a global ID card. For 2-Acetyl Thiazole, 24295-03-2, this unique code turns a tongue-twister chemical name into something reliable for tracking, research, or import forms. I’ve seen how these numbers take away confusion. With a budget tight on time, skimming technical sheets for the CAS entry gets right to the point, slicing through variations in spelling or language.
In kitchens and food plants, micrograms make the difference between a food that’s irresistible and one that might trigger worry. Traceability matters for more than sales figures. 2-Acetyl Thiazole often shows up in everything from popcorn flavoring to baked goods. Regulators want nothing left to guesswork, so that precision falls to CAS numbers. If a safety recall flares up or regulations shift, knowing which exact batch holds which chemical means less chaos and more control. Reports from the European Food Safety Authority point out the importance of compound tracking for food additives, especially since even safe flavors can raise allergies or health concerns at high doses.
Consumers keep demanding to know what’s inside their snacks, and for good reason. Experienced cooks and buyers can’t easily scan packaging for unpronounceable chemical names, but many major ingredient suppliers post CAS numbers on spec sheets. This isn’t just for show. It builds trust from the ground up. In one project for a small company creating plant-based meat alternatives, confusion over flavoring sources brought serious delays until everyone agreed to use CAS numbers for every ingredient. That sort of clarity cut down the headaches, smoothed out safety reviews, and reassured buyers.
A world full of global trade and complicated supply chains puts pressure on everyone, from farmers to food engineers. Using CAS numbers like those for 2-Acetyl Thiazole strengthens the foundation. Beyond the technical side, education plays a role too. Industry should do more to share guides on reading ingredient lists and understanding chemical IDs. Everyone, from high school science students to home cooks, benefits from clear labeling—especially as new food tech arrives. The move toward greater transparency isn’t quick, but it offers a long-term payoff in safety, quality, and peace of mind.
Even small details, like a single CAS number, create real-world effects at every step, supporting better decisions, higher safety standards, and a trust that runs both ways, from supplier to consumer.
2-Acetyl thiazole brings a roasted, nutty aroma that shows up in plenty of flavors and fragrances. Over the years in the lab, I’ve realized it’s a compound you respect — not just for its scent, but also for how quickly it can go bad if left neglected on a shelf. Years of working with food ingredients and flavor chemicals has taught me that chemistry rewards care and preparedness.
With volatile, sulfur-rich compounds like this, the enemy is often the air itself. Exposing an open bottle will leave you with weaker aromatics and strengthened off-odors after just a matter of weeks. You also get unpleasant surprises when moisture sneaks in and the chemical starts to form impurities. If you plan on using 2-acetyl thiazole in food or research, this sort of careless storage simply won’t cut it.
In my own work, I’ve seen the difference between ingredients kept under proper conditions and those kept casually. A refrigerator or, even better, a cold room helps keep this compound fresh longer. Storing it somewhere cool—ideally under 8°C—slows any chemical breakdown or off-odor formation. Heat will do the opposite, so keep it out of places like supply closets near dryers or under direct sunlight.
A tightly closed amber glass bottle does wonders. I always avoid plastics for sulfur flavors, since their volatile components can react or even slowly diffuse out of cheaper containers. Amber glass cuts down on light exposure, a big culprit in chemical change, and helps preserve the original profile of the ingredient.
Don’t underestimate the impact of air. As soon as you open a container, make a habit of closing it immediately after use. For larger labs or flavor houses, moving contents to smaller vials can help reduce the amount of air sitting in the bottle. I’ve seen more than one batch spoiled from a careless pour and a long, open sitting on the workbench.
Sulfur compounds leave a mark on almost everything they touch. If you work in a shared environment, double-bagging and secondary containment prevent the aroma from escaping and lingering in storage spaces. I’ve done side-by-side tests and found that this simple technique makes a world of difference in keeping cold storage pleasant for everyone.
Make sure your workspace has good ventilation since some people develop headaches or nausea from too much sulfur vapor. Label your bottles clearly, not just with the chemical name but also with safety notes and the date opened. It takes seconds, but helps avoid confusion and cross-contamination—problems that tend to show up as mystery odors months down the line.
Reliable results depend on small acts of discipline: storing bottles away from light, locking the cap every time, and tracking inventory. If a batch starts looking cloudy, changes color, or smells different, don’t risk it—dispose of it following local rules. In my own practice, these habits save time, money, and headaches. Attention turns a fragile ingredient into a reliable tool.
By setting a standard and sharing knowledge with team members, you avoid slip-ups that cost more than just product loss. Safe, thoughtful storage preserves not only the chemical itself but also trust in your food or research project. These aren’t just best practices—they’re the foundation of every successful lab and production kitchen I’ve visited.
| Names | |
| Preferred IUPAC name | 1-(Thiazol-2-yl)ethan-1-one |
| Other names |
2-Acetyl-1,3-thiazole 2-Thiazole methyl ketone 2-Acetythiazole 2-Acetylthiazol Methyl thiazolyl ketone |
| Pronunciation | /tuː əˈsiːtɪl θaɪˈæzoʊl/ |
| Identifiers | |
| CAS Number | 24295-03-2 |
| Beilstein Reference | 104132 |
| ChEBI | CHEBI:51723 |
| ChEMBL | CHEMBL16216 |
| ChemSpider | 157401 |
| DrugBank | DB04280 |
| ECHA InfoCard | 100.016.392 |
| EC Number | 2.5.1.6 |
| Gmelin Reference | 8798 |
| KEGG | C14524 |
| MeSH | D000197 |
| PubChem CID | 6985 |
| RTECS number | XN8575000 |
| UNII | X5R4WR758B |
| UN number | UN number: "2810 |
| CompTox Dashboard (EPA) | DTXSID9020605 |
| Properties | |
| Chemical formula | C5H5NOS |
| Molar mass | 155.21 g/mol |
| Appearance | Pale yellow to yellow liquid |
| Odor | nutty, popcorn, roasted |
| Density | 1.18 g/cm3 |
| Solubility in water | slightly soluble |
| log P | 1.18 |
| Vapor pressure | 0.021 mmHg (25°C) |
| Acidity (pKa) | 12.9 |
| Basicity (pKb) | 12.86 |
| Magnetic susceptibility (χ) | -58.95×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.554 |
| Viscosity | 2.345 cP (20°C) |
| Dipole moment | 2.81 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 184.6 J/mol·K |
| Std enthalpy of formation (ΔfH⦵298) | -93.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2043 kJ·mol⁻¹ |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS05, GHS07 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. |
| Precautionary statements | P261, P264, P271, P272, P273, P280, P301+P310, P302+P352, P304+P340, P305+P351+P338, P308+P311, P312, P321, P330, P332+P313, P333+P313, P337+P313, P362+P364, P403+P233, P405, P501 |
| Flash point | 95°C |
| Autoignition temperature | 210 °C |
| Lethal dose or concentration | LD50 (oral, rat): 1600 mg/kg |
| LD50 (median dose) | LD50 (median dose): 790 mg/kg (oral, rat) |
| NIOSH | DA8050000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 5.00 |
| Related compounds | |
| Related compounds |
2-Acetyl-4-methylthiazole 2-Propionylthiazole Thiazole 2-Methylthiazole 2-Ethylthiazole 2-Formylthiazole |