People have studied heterocyclic compounds for generations, but 2-Acetyl Pyrrole has carved out a unique spot thanks to its warm, breadlike aroma and its role in the chemistry of flavor. The compound first drew attention in the 1920s as food scientists hunted for the molecules behind the scent of baking bread and roasted coffee. Chemists found it turning up from the Maillard reaction, where sugars and amino acids meet under heat, forming flavors that define home-cooked meals. Since then, discoveries poured in about its extraction from foods like popcorn and its natural occurrence in tobacco smoke, giving shape to a wider understanding of both its benefits and its risks.
2-Acetyl Pyrrole brings a characteristic, nutty aroma to the table, giving real depth to flavors in everything from processed foods to perfumes. Food labs use trace levels to recreate authentic bread, coffee, or chocolate notes in products. It also finds a home in fine fragrance creation, giving top “gourmand” notes a warm, cozy backdrop that stays true to the original experience. If you’re eating anything flavored to taste like it came straight out of a bakery, there’s a decent chance this compound helped shape the experience.
This chemical pours out as a colorless to pale yellow oil with a melting point hovering around -17°C and a boiling point near 197–199°C at standard pressure. Its molecular formula, C6H7NO, gives a mass of about 109.13 g/mol. 2-Acetyl Pyrrole dissolves well in common organic solvents but doesn’t play well with water. That hydrophobic streak amplifies its ability to hang in fatty matrices and essential oils. Its vapor carries an unmistakable scent, which easily explains the compound’s track record in flavor-enhancing chemistry.
Buyers and regulatory agencies keep a close eye on purity, with reputable sources supplying anything from 98% up to analytical-grade batches. Labels must spell out hazards, including its possible irritant action on skin or eyes, clear instructions for PPE, and appropriate storage conditions for a compound prone to oxidation. SKU listings often mention “2-AP,” “2-Acetyl-1H-pyrrole,” or “Methyl 2-pyrrolyl ketone” for clarity in trade and research settings.
Most large-scale production uses acetylation of pyrrole under Friedel-Crafts conditions. Chemists mix pyrrole with acetic anhydride (or acetyl chloride) in the presence of a Lewis acid such as aluminum chloride, then isolate the product through distillation. Careful temperature and moisture controls matter here, since pyrrole’s electron-rich ring wants to react in all directions, and even a little water can throw things off. In labs, milder routes remove the need for corrosive catalysts, making for a cleaner prep but scaling up remains a challenge.
Researchers find 2-Acetyl Pyrrole to be a versatile chemical handle. Its reactive acetyl group opens doors for further transformations, including aldol reactions, reductions to alcohols, or substitutions with nucleophiles. On the ring side, care must be taken in any aromatic substitution since the pyrrole core is sensitive and can rearrange under harsh conditions. Synthetic chemists often make analogs by tweaking the side chain or the heterocycle, building libraries for pharmaceuticals or new food additives. Each tweak brings a shift in aroma, boiling point, or biological properties, giving teams tools to fine-tune end-use products.
Across the globe, you’ll see listings as 2-AP, 2-acetyl-1H-pyrrole, or methyl 2-pyrrolyl ketone. CAS 1072-83-9 tags shipments between distributors, and flavor houses sometimes market it as “popcorn ketone” for instant recognition among buyers looking to spark up freshly baked flavors. Found as a natural isolate, it’s often listed under “natural flavoring complex,” pushing product developers to double-check their ingredient sources.
National food authorities and workplace safety frameworks keep a steady eye on 2-Acetyl Pyrrole, given its volatility and mild irritant action. Safe handling calls for gloves, goggles, and good ventilation. If it spills, wiping up promptly and disposing of rags properly will help avoid inhaling fumes. For transport, keeping drums or bottles tightly sealed, away from ignition sources, follows mandatory hazard labeling by international air and maritime codes. In a lab, built-in fume extraction and splash protection can make all the difference.
Food scientists turn to 2-Acetyl Pyrrole for its nutty, malted, and roasted notes in snacks, instant coffee, baked goods, and chocolate. Its signature aroma gives authenticity to processed foods that need to capture the essence of hearth and home. Beyond food, perfumers use trace levels to create bakery-themed or sweet fragrances. Chemists track it as a flavor marker in quality control, while tobacco companies monitor it in smoke research. Very low regulatory thresholds keep its use mainly within controlled food and fragrance blends, rather than direct or bulk exposures.
In the last decade, technical teams have dug into new routes that use greener catalysts for making 2-Acetyl Pyrrole to trim costs and cut hazardous waste. Computational chemists study how changing the pyrrole ring tweaks aroma thresholds, helping food scientists dial flavors closer to natural profiles. Analytical labs lean on chromatography to pick out this molecule down to parts per billion, helping trace flavor development in products. Universities are keen to explore how such heterocycles form naturally in roasted foods to inform healthier, more authentic food design.
For all its flavor charm, 2-Acetyl Pyrrole raises some flags if mishandled. Lab studies suggest modest acute toxicity in animal models if consumed or inhaled in large doses, comparable to other food-borne flavorants. Irritation comes from direct contact with eyes or mucus membranes. Chronic exposure at workplace levels shows limited evidence of harm, though most studies deal with higher doses than common in food. Regulatory panels limit allowable amounts strictly, enforcing food-grade standards and transparent labeling to prevent accidental overuse. Ongoing work keeps a watch on metabolites and breakdown products to catch unexpected risks.
Interest in natural and “clean label” flavors sets up 2-Acetyl Pyrrole as a long-term staple in food chemistry. Teams are working on biosynthetic methods using enzyme cascades that mimic the Maillard reaction, letting factories make it from simple sugars and amino acids under controlled conditions that use less energy and generate fewer byproducts. As consumers dig deeper into the science of flavor, transparency around sourcing and safety will keep this compound in demand for both traditional and plant-based foods. With ever-better detection tools, research into low-level toxicology keeps the industry honest. If science finds safer, faster, or more sustainable ways to produce and capture the nutty, comforting essence of this molecule, its world of uses is only going to grow.
Take a walk past any bakery, and you might catch a whiff of freshly baked bread. That toasty, nutty scent often comes from a little compound called 2-acetyl pyrrole. This molecule steps into a surprising number of roles, starting with food and flavor. Anytime someone bites into a chocolate bar, sips on roasted coffee, or enjoys grilled meat, there’s a good chance 2-acetyl pyrrole plays a subtle part in the experience. I remember working in a small coffee shop, and every time we roasted a fresh batch of beans, the aroma changed the whole place—and it wasn’t just for decoration. That feeling of coziness connects right back to chemistry happening in the roaster.
Food scientists lean on 2-acetyl pyrrole to boost natural flavors. Because the baking and roasting process naturally creates this molecule, recreating that same feel in processed foods becomes possible with a careful touch of 2-acetyl pyrrole. It gives products like breakfast cereals, baked snacks, and some plant-based meats a more convincing “cooked” scent. Without this, many foods end up tasting flat or artificial even with the most advanced recipes.
It doesn’t stop with food. Perfumers like 2-acetyl pyrrole for its warm, nutty note, which blends well in woody and tobacco scents. The molecule brings depth, helping to round out fragrances so they don’t overwhelm the nose. Over the years, technical uses have grown, too. In chemical research, 2-acetyl pyrrole serves as a building block for bigger, more complex molecules. Some dye and pharmaceutical work relies on this compound, because its structure makes certain chemical reactions easier to pull off.
Like any flavor enhancer, safety matters. 2-Acetyl pyrrole lands on the FEMA GRAS (Generally Recognized As Safe) list for food use in small amounts, so manufacturers must keep doses low. Most foods don’t need much—a little goes a long way. Overexposure, especially in industrial settings, does raise questions. Workers handling concentrated forms should follow protection guidelines, because smelling something pleasant doesn’t always mean it’s risk-free.
The scale of production means keeping a close eye on sourcing and waste matters, too. Chemical synthesis often creates by-products, and companies have a responsibility to clean up after themselves. Research into greener manufacturing continues, with some teams experimenting with renewable sources and waste-reducing techniques. If these practices become standard, both people and the planet see benefits. Transparency in labeling also helps. Most shoppers rarely recognize flavor molecules on a label, but more clear ingredient information leads to better choices at the grocery store.
2-Acetyl pyrrole reminds us that the best parts of food—flavor, aroma, comfort—link back to molecules and careful design. Every time I bake bread at home and catch that rich, almost roasted smell, I see a little of the science behind simple pleasures. With smart use and responsible industry practices, this compound keeps making our daily rituals more enjoyable, one bite or sip at a time.
2-Acetyl pyrrole presents a curious little molecule, both for how it’s put together and where it shows up in daily life. The molecular formula, C6H7NO, lays out its basic recipe—a combination of carbon, hydrogen, nitrogen, and oxygen atoms. The backbone of this molecule stands out because it forms from a five-membered pyrrole ring. This ring is made up of four carbons and one nitrogen, which is a common scaffold in natural and synthetic chemicals alike.
The key structural twist? An acetyl group connects to the second position of the pyrrole ring. That means you’ll find a carbonyl (C=O) attached to a methyl (CH3), bonding right onto the ring next to the nitrogen atom. Chemists call this position the “2” spot, tracing counterclockwise from the nitrogen.
Here’s where things get interesting. This arrangement gives 2-acetyl pyrrole a distinct aroma, something you can actually relate to if you’ve ever caught a whiff of freshly baked bread or roasted coffee. It’s not just a sterile lab curiosity—this molecule pops up in foods after baking or roasting thanks to the Maillard reaction. There’s a reason industrial flavor houses track its behavior carefully. Even in trace amounts, the structure guarantees a nutty, warm note that’s hard to fake.
Food scientists pay close attention to molecules like 2-acetyl pyrrole. Safety stands front and center for any chemical meant for flavoring, and fortunately, toxicology studies on this compound have not raised red flags at concentrations usually found in food. The Food and Drug Administration includes it on lists of ingredients considered safe for use in flavors. This doesn’t give anyone a license to dump it indiscriminately, though. Consistent oversight, thoughtful blending, and close monitoring always stay essential.
The story hardly stops at flavor. In the pharmaceutical world, that pyrrole ring crops up often as a building block. Scientists modify and tweak structures like 2-acetyl pyrrole to hunt for new medicines. Early-stage research highlights these pyrrole derivatives for their potential in cancer, anti-inflammatory, and antimicrobial drugs. Getting the structure right here means better outcomes, fewer side-effects, and more options for patients. Even if most folks never hear the name, new treatments could trace their roots back to molecules like this.
The structure of 2-acetyl pyrrole gives both flavor and function. It brings out hidden tastes in foods and shows up as a key stepping-stone in medicine research. Chemists and food scientists must keep collaborating to ensure both safety and innovation. More detailed analysis—using methods like spectrometry, crystallography, and chromatography—helps confirm where this molecule shows up in products and guarantees it meets purity standards. New food safety checks, ongoing discussions among regulators, and clear labeling keep the public in the loop.
The story of 2-acetyl pyrrole runs through labs, kitchens, and even hospitals, tying together hard data and everyday experience. Knowing a bit about the way these atoms fit together sheds light on why flavors and medicines turn out the way they do.
2-Acetyl pyrrole pops up on the ingredients list of foods and perfumes way more often than most people might think. This compound smells a lot like baked bread and roasted nuts, which means it helps bring that extra depth and coziness to anything from chocolate bars to fancy colognes. I’ve sniffed enough candles and flavored treats to recognize its signature aroma, and there’s no denying its allure.But good smells come with good questions, and the most important one is whether 2-acetyl pyrrole earns its spot in our diets and daily products without health worries.
The US Food and Drug Administration lists 2-acetyl pyrrole as "Generally Recognized As Safe" (GRAS) for use in foods. This label shows up only after plenty of toxicology data stacks up to say that a substance causes no harm at expected levels. Studies run by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) have not flagged it as a hazard when used in tiny amounts, the way food makers do.
This ingredient isn't new. Bakeries and candy makers have turned to 2-acetyl pyrrole for decades to boost flavor, and major food flavor authorities—including FEMA (Flavor and Extract Manufacturers Association)—still keep it on their “okay” list. They do not turn a blind eye to consumer health, especially after famous missteps in food science history.
Just because a chemical makes bread taste toastier does not give a free pass for unlimited use. There’s a real difference between a pinch in a snack bar and a dump truck full. At high concentrations, 2-acetyl pyrrole can cause skin or eye irritation, the way many concentrated aroma compounds do. Nobody recommends applying pure liquid to skin, eyes, or breathing it in directly. Perfume blenders know this and use strict guidelines for how much goes in a bottle.
Research on chronic effects in humans is not deep. Fifteen years in a flavor lab taught me that safe use often depends on guardrails—clear labeling, keeping ingredient levels ridiculously low, and recognizing when one person’s treat might trigger another person’s allergy. Some people have extra sensitivity, but no public allergy alerts or toxicity recalls have trailed 2-acetyl pyrrole over decades of use.
People care more about what’s going into products than ever before. Companies can step up by sharing more detail about the ingredients they use, down to the specific flavor molecules like this one. If someone wants to avoid all artificial or “lab-made” flavors, they deserve to have that choice—and clear information is the only way to support that. Retailers should make that information easy to spot, not buried at the end of a label.
The best path forward is clear. Set limits based on updated research, keep an eye on new studies, and focus on transparency in labeling. Avoiding excessive, untested exposure protects everyone. And for folks who prefer “all natural,” having honest labels and options matters just as much as scientific consensus. This approach builds trust and keeps the conversation honest. That’s the only way ingredient safety—and product quality—grow together.
2-Acetyl pyrrole pops up in quite a few industries because its smell blends easily into flavors and fragrances. I remember walking into labs where it sat in small amber bottles, tucked away from direct sunlight. You never want it in a spot that feels too warm or moist. Heat and moisture can mess with the compound, causing it to clump or degrade, and nobody wants to lose money because a chemical gets ruined on the storage shelf.
Many chemists, including myself, prefer keeping 2-acetyl pyrrole in airtight containers. Glass remains a top pick. I’ve noticed plastic sometimes reacts, especially if you’re not sure what kind you’re using. The storeroom stays cool, usually at room temperature or a little lower, but not freezing. Any accidental freeze-and-thaw cycles affect the quality and could cause crystals to form or change. Shelves should stay dry, and every container must wear a clear label—date received, lot number, and manufacturer.
Even though this might sound straightforward, mistakes happen. Someone once left a bottle near a window at noon in July. After a few days, a strong smell filled the hallway. We traced it back and realized how quickly heat spoils it. That incident reinforced the lesson: sunlight and heat are quiet wreckers, not just for 2-acetyl pyrrole but for most sensitive lab stocks.
No matter the industry, gloves always come out before handling the substance. Some companies standardize nitrile or neoprene, which gives solid protection. I avoid latex when acids or organic compounds are involved. Spills can be a nightmare, especially if a workspace doesn’t have proper ventilation. A whiff outside a fume hood stings more than you’d think. Breathing protection should sit within reach—proper masks or respirators, not just flimsy dust masks.
Measuring and weighing work best on clean, dedicated benches. Open flames or ignition sources should stay far away. I’ve seen someone light a Bunsen burner across the bench, forgetting the volatility of compounds nearby. A split-second lapse nearly started a fire. No matter how busy a schedule, skipping good habits invites disaster.
Handling training keeps accidents low. New staff watch older hands and learn to check labels and logs before even touching anything. Chemical safety sheets shouldn’t gather dust. Refreshing this knowledge every six months lifts awareness. Too often, complacency creeps in after months of quiet routine. That’s when spills happen or mistakes multiply.
Labels save lives. Every container, every shelf, and every log needs to make clear what’s inside and how old it is. Cross-contamination happens when labels wear and nobody remembers what’s inside. Tracking inventory helps nobody run out—or worse—substitute the wrong chemical in a hurry. Many industries, including food-flavor labs where I once worked, rely on these checks. Skipping them leads to ruined flavor batches or contaminated products, which travel down the whole production chain.
Regular checks by supervisors catch sloppy storage. Simple habits, like wiping down containers after use and never returning unused powder to the original jar, go a long way. Spill kits must always be stocked and close to handling areas. I’ve seen more than one crisis turn manageable with everyone knowing where to find sand, absorbent pads, and proper disposal bins.
In the end, keeping 2-acetyl pyrrole safe always rests on clear habits, teamwork, and practical training. The more we rely on each other and good routines, the fewer headaches we face later.
2-Acetyl pyrrole shows up as a pale yellow to amber liquid at room temperature. I’ve seen it pour almost like lightweight syrup. You catch a roasted, nutty aroma from it that reminds many of toasted bread or popcorn. That scent actually helps flavorists. They turn to this compound for baked and cocoa notes in foods or fragrances. The color and smell often give away its identity far quicker than a chemical label does in a busy lab.
This compound mixes well with alcohol and diethyl ether but struggles with water. In practice, this means if you try to dilute it in water, you’ll see it float or separate out. But drop it into ethanol or other organic solvents, and it blends right in. In the lab, I've watched how it stays reasonably stable at room temperature. Let it sit in the sun or heat it up, though, and the quality drops. You might see discoloration or degradation. Chemists avoid plastic containers. Glass wins out to keep 2-acetyl pyrrole’s character unchanged.
Looking at its structure, there’s a five-membered pyrrole ring linked with an acetyl group. The rings and branches in this molecule define how it reacts. It has a molecular weight around 109 grams per mole and the formula C6H7NO. What I’ve learned is that its double bonds and lone pairs make it eager to react in the presence of acids, bases, or oxidizing agents. This helps chemists in the production of pharmaceuticals and flavors, where controlled reactions need a reliable starting material.
2-Acetyl pyrrole boils at about 199°C and melts just below room temperature. In real-world settings, this means you can handle it as a liquid at most indoor conditions. Its vapor pressure is low, so it doesn’t evaporate as quickly as other solvents I’ve worked with, but heating brings out more of its aroma. That’s why roasting or baking releases these familiar smells in the kitchen or factory floor.
Working safely with 2-acetyl pyrrole means understanding its moderate toxicity and the risks of skin or eye irritation. Breathing in too much vapor can bring headaches. Many workplaces set up good ventilation and keep this compound capped and stored in dark, cool areas. As someone who has handled a range of aromatic compounds, I see 2-acetyl pyrrole as no more hazardous than other small organics, but gloves and goggles remain the best defense. Routine training limits most exposure issues.
Food scientists, perfumers, and drug developers turn to 2-acetyl pyrrole for its reliable properties. I’ve seen it improve everything from chocolate bars to lifesaving drugs, all because it holds up under predictable conditions and brings quality aroma. The legal side matters, too. In most countries, regulators approve it in tiny amounts for flavoring—once purity checks out and labs confirm there’s no hidden contamination. Ongoing research explores greener ways to synthesize this compound, cutting down on waste and energy. That shift promises to make future batches safer for both people and the planet.
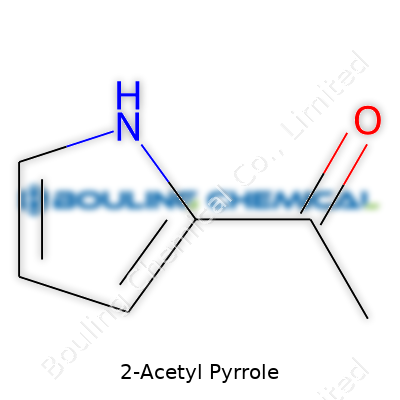
| Names | |
| Preferred IUPAC name | 1-(1H-pyrrol-2-yl)ethan-1-one |
| Pronunciation | /tuː əˈsiːtɪl ˈpɜːr.oʊl/ |
| Identifiers | |
| CAS Number | 1072-83-9 |
| Beilstein Reference | 1209243 |
| ChEBI | CHEBI:18936 |
| ChEMBL | CHEMBL33063 |
| ChemSpider | 57739 |
| DrugBank | DB04268 |
| ECHA InfoCard | 100.008.765 |
| EC Number | 1202-34-2 |
| Gmelin Reference | 802931 |
| KEGG | C06504 |
| MeSH | D000198 |
| PubChem CID | 6998 |
| RTECS number | UF3675000 |
| UNII | H7U49U79R6 |
| UN number | UN1989 |
| Properties | |
| Chemical formula | C6H7NO |
| Molar mass | 107.12 g/mol |
| Appearance | Light yellow to brown liquid |
| Odor | caramel; nutty; popcorn |
| Density | 1.08 g/cm3 |
| Solubility in water | Slightly soluble |
| log P | 0.32 |
| Vapor pressure | 0.019 mmHg (25°C) |
| Acidity (pKa) | 13.8 |
| Basicity (pKb) | 6.86 |
| Refractive index (nD) | 1.531 |
| Viscosity | 1.106 cP (20°C) |
| Dipole moment | 2.83 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 116.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -45.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2224.8 kJ/mol |
| Pharmacology | |
| ATC code | A11HA |
| Hazards | |
| Main hazards | Harmful if swallowed, causes serious eye irritation, causes skin irritation |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. |
| Precautionary statements | Precautionary statements for 2-Acetyl Pyrrole: "P261, P305+P351+P338, P304+P340 |
| NFPA 704 (fire diamond) | 2-2-0 Health:2 Flammability:2 Instability:0 |
| Flash point | 94°C |
| Autoignition temperature | 245 °C |
| Explosive limits | 2.1–15% (in air) |
| Lethal dose or concentration | LD₅₀ (oral, rat): >2000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral-rat LD50: 2000 mg/kg |
| NIOSH | BX8925000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | REL (Recommended): 0.1 ppm |
| Related compounds | |
| Related compounds |
Pyrrole 2-Formylpyrrole 2-Methylpyrrole 2-Ethylpyrrole 3-Acetylpyrrole N-Methylpyrrole Pyridine Indole |