2-Acetyl pyrazine’s story begins in the age of flavor chemistry. Originally, the chemical world stumbled onto this compound while digging for the secrets behind roasted and baked aromas. Back in the 1970s, as analytical tools sharpened, scientists found that even tiny amounts give foods that toasty, cracker-like scent you recognize from fresh-baked bread or roasted nuts. This breakthrough helped food scientists pinpoint why certain snacks and cereals taste so good. Over time, companies started to synthesize 2-acetyl pyrazine to keep up with growing demand, especially as baked products became an industrial staple. Today, it finds its way into everything from snack foods to ready meals, evidence of how food chemistry and industry have moved together.
2-Acetyl pyrazine belongs to the pyrazine family, notable for their rich, nutty notes. Anyone who’s ever opened a box of popcorn or torn into a cookie straight from the oven has encountered the unmistakable aroma. In its pure form, this compound comes as an off-white to pale yellow crystalline solid. Manufacturers appreciate its potency; even at concentrations as low as a few parts per billion, it transforms bland base materials into foods with immediate aroma impact. Its shelf-stable nature and ease of handling support both large-scale production and experimental use in R&D labs exploring flavor innovation.
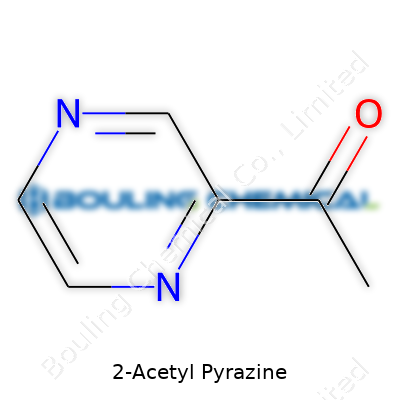
2-Acetyl pyrazine has a molecular formula of C6H6N2O, clocking in with a molecular weight around 122.12 g/mol. Its melting point generally sits between 80–84°C, and it offers low solubility in water but dissolves readily in organic solvents like ethanol and diethyl ether. Its structure includes a pyrazine ring substituted with an acetyl group, which makes it particularly reactive toward nucleophilic additions. As a result, labs working with this material see different behaviors based on the solvent or application. The low odor threshold gives formulation specialists quite a bit of leeway, since very little is needed to deliver the right aroma.
Exacting standards govern 2-acetyl pyrazine, since it regularly ends up in things people eat. Most suppliers guarantee a purity not less than 98%, with the rest made up by trace isomers or minor contaminants. Packaging often involves secure, food-grade drums lined with inner bags to keep out moisture and contaminants. Industry labeling sticks close to international agreements such as IUPAC naming, hazard warnings under GHS standards, and explicit concentration details. Food safety authorities, like the US FDA and the EU’s EFSA, require clear documentation for origin and batch history, tracking each shipment from plant to customer.
In industry, synthesizing 2-acetyl pyrazine starts with a pyrazine core, often derived from diketones like glyoxal and acetyl derivatives. Commonly, chemists use an acylation reaction, where acetyl chloride and pyrazine mix under carefully controlled conditions, frequently in the presence of catalysts and under chilled temperatures to favor the correct isomer. Some pioneering methods use microwave assistance or solvent-free techniques to improve yields and reduce byproduct formation. Manufacturers gravitate toward greener routes, optimizing for both cost and minimum impact on the environment, a trend nudged along by stricter waste regulations across Europe and the United States.
2-Acetyl pyrazine displays versatility in chemical synthesis. The acetyl group introduces reactivity that allows for further modification. Chemists exploit this by developing pyrazine-based flavor enhancers or by linking the molecule to other flavor-active fragments. This compound stands up well under normal food processing temperatures, though it may degrade at high heat, leading to pyrazine ring opening or fragment loss. Some advanced labs work with halogenation or alkylation to build structurally similar compounds that deliver unique nuanced aromas. The breadth of reaction possibilities keeps this compound at the center of both industrial synthesis and cutting-edge flavor research.
2-Acetyl pyrazine often appears under a handful of names in industry circles. Sometimes it goes by the systematic tag 1-(pyrazin-2-yl)ethan-1-one. It may also pop up on labels as acetylpyrazine, or with manufacturer's codes unique to large flavor houses. These names may not mean much to the end consumer, but accurate identification matters for regulatory affairs and safety data sheet management. The synonym game becomes especially important when a company sources from multiple suppliers or ships product between regions using different chemical inventories, such as REACH in the EU or TSCA in the United States.
Any operation working with 2-acetyl pyrazine keeps a close eye on safety guidelines. Inhalation of dust, skin contact, or accidental ingestion raise acute concerns, though the compound is not highly toxic under normal usage levels. Facilities keep ventilation systems running and equip staff with gloves, goggles, and sometimes dust masks. Fire response protocols come standard, given pyrazines' flammability. All plant staff learn to handle spills quickly, using absorbent materials and proper waste bins. Regulatory frameworks require regular updates to safety data sheets (SDS), which outline everything from first aid steps to recommended disposal processes. Firms conduct routine audits and emergency drills, driven both by insurance and the real-world need to keep teams safe.
Bakers, confectioners, snack-makers, and beverage companies rely on 2-acetyl pyrazine to lift recipes above the ordinary. Its signature roasted quality strengthens chocolate, nuts, and cereal flavors, making it a favorite for flavorists chasing that elusive just-roasted experience in everything from granola bars to whiskey. Tobacco product manufacturers blend it to impart depth and warmth, and some pet food producers find it boosts palatability for finicky eaters. A handful of aroma chemists in perfumery experiment with it, searching for new base notes, though it doesn’t see widespread adoption there. Over the years, the ingredients sector has counted on its stability and performance across a spectrum of pH, making it a mainstay in both liquid and dry production lines.
Academic institutes and flavor houses pour resources into deepening the understanding of pyrazine analogs. R&D labs probe the relationships between structure and scent by designing new derivatives, hoping to unlock richer, more specific aromas for fine-tuning everything from artisan chocolates to plant-based meat. Sensory analysis and gas chromatography studies turn up new knowledge about how people perceive these molecules. On the consumer trend side, much of the recent R&D zeroes in on natural sourcing, hunting for microbes or roasting processes that yield natural-identical 2-acetyl pyrazine at scale. The push for clean-label solutions and minimal-processing has ramped up investments across the field.
Most toxicological data points to low acute toxicity for 2-acetyl pyrazine in standard food use. Studies in rodents and cell cultures show high safety margins, with regulatory reviews by bodies like the Joint FAO/WHO Expert Committee on Food Additives supporting this view. Nonetheless, safety teams keep an eye on long-term effects and interaction with other dietary elements, especially as consumption patterns shift and new delivery formats emerge. As with many food ingredients, the adage ‘the dose makes the poison’ applies. Regular re-evaluation by global agencies ensures updated guidance for both health professionals and manufacturers, a safeguard as usage widens into emerging sectors.
As food culture changes, 2-acetyl pyrazine stands at the crossroads between tradition and tomorrow. The fast growth of plant-based foods and alternative proteins drives up demand for powerful, authentic, and label-friendly flavor compounds. New biotechnological approaches shine a light on fermentative routes, promising reduced carbon footprints and potentially unlocking new regulatory approvals in markets that value non-synthetic origins. Digital modeling and AI increasingly play roles as researchers seek to predict and optimize flavor perceptions before field testing. With climate pressures and resource constraints shaping industry choices, next-generation production methods and sustainability certifications become part of the fabric. In all this, one molecule and its distinctive roasted warmth keep its value, adapting alongside the industry that gave it life.
Try to picture a lazy morning, someone toasting a handful of nuts in the kitchen. That rich, nutty scent reminds most folks of comfort food—or home. Hidden beneath this aroma is a compound that gives so many snacks that “roasted” edge: 2-acetyl pyrazine. I first ran into it while trying to track down the reasons some store-bought cereals smelled almost alive, like someone just broke out a skillet and started roasting cornflakes. It turns out this chemical shapes the flavors and aromas in places you’d never expect.
Across the food industry, the stuff acts like a magic wand for taste buds. It goes into the recipe for anything that aims for a roasted or baked note—think popcorn, pretzels, chocolate, or corn chips. 2-acetyl pyrazine fools you into believing something’s fresh from the oven, even if it arrived in a sealed bag from across the country. I’ve learned from flavorists that a pinch of this compound in their toolbox lets them create nutty flavor profiles out of even the dullest ingredients.
The food world moves fast to keep up with changing taste trends and regulatory rules. More governments have begun looking at what goes into additives, pushing for more transparency and safer formulas. 2-acetyl pyrazine has gone through food safety assessments, revealing it doesn’t pose risks in standard dosages used for flavoring. That said, people want ingredients they can trust, and some are still wary about anything that comes with a chemical name. Natural sources—like roasted grains or coffee—carry this compound too, so it’s not always some artificial concoction.
Dig deeper and this molecule also shows up in perfumes and personal care. It provides a warm, comforting base note, echoing toasted nuts or even buttery baked goods. The scent gets woven into candles or lotions, those tiny touches that make a bathroom feel welcoming. I remember working on a project where we tried to make a scented soap line reminiscent of an old-fashioned bakery, and nothing triggered that “fresh bread” feeling like 2-acetyl pyrazine.
There’s another side worth talking about—the vaping industry discovered this molecule’s flavor punch. Some e-liquid brands use it in “nutty” or “toasty” flavors. But concerns mount here, given the unknowns around inhalation versus eating. The U.S. Food and Drug Administration has only started catching up with these developments. While ingestion looks safe within limits, inhalation brings up more debate. We’ve seen studies point out a lack of long-term data about its effects in vapor, which calls for tighter rules and open labels.
Food science and flavor chemistry keep searching for new ways to make food taste better without sacrificing safety or honesty. As a food lover, I believe clear labeling and smart regulation make a real difference. People want to know what’s in their food and their air—whether it brings them back to a cozy kitchen or just boosts the taste of a midnight snack. The big picture here: anyone using ingredients with complex names should open up about their purpose and safety. Better trust means better choices for everyone at the table.
You ever pick up a bag of roasted nuts or chocolate and catch that toasty, nutty aroma that screams “fresh baked”? There’s a good chance 2-acetyl pyrazine plays a quiet role in that flavor. This molecule gives snacks, sweets, and even some vape liquids a warm, roasted note that feels like comfort food.
You might wonder, though, if this flavor enhancer belongs in your diet. 2-acetyl pyrazine didn’t fall from the sky into coffee and chocolate. Food scientists added it for its impact. Regulatory bodies like the U.S. FDA look at these ingredients closely. The Flavor and Extract Manufacturers Association (FEMA) includes 2-acetyl pyrazine on its GRAS (Generally Recognized As Safe) list. That means they found enough evidence that small, typical food-use levels appear safe.
Most safety conversations about food additives start with the dose a person actually eats. Studies on 2-acetyl pyrazine looked at how animals handled it. At low doses—the sort found in flavored foods—animals didn’t show toxic effects or obvious health issues. In trials with higher doses, where you’d be eating far more than any average serving, researchers saw some negative reactions. For a real-world comparison, you’d need to eat unrealistic amounts of the foods that contain it to hit those levels.
The European Food Safety Authority (EFSA) also surveyed available evidence and did not spot significant risks at the low concentrations found in foods. With daily diets containing trace levels, regular consumers won’t reach problematic amounts. The molecule also occurs naturally in cooked foods, roasted coffee, popcorn, and grains. Synthetic production came about because getting enough from natural sources never matched commercial demand.
People can get nervous about any specialty ingredient with a complex chemical name. I understand the concern—anything unfamiliar sparks questions. In my kitchen, I pay attention to labels. It’s about being informed, not just being afraid of the unknown. When I learned more about how flavor companies use 2-acetyl pyrazine, I stopped picturing chemical vats and started seeing it as an echo of what naturally happens during roasting or baking.
The real issue comes with bigger doses, often outside normal food situations. For example, heavy use in e-cigarette liquids stirs up more debate. Some doctors warn inhaled exposure isn’t the same as digestion, so the safety profile looks murkier there. Sticking to food-based products keeps things in the well-studied realm.
If you’re sensitive to additives, always keep an eye on ingredient lists. Nothing beats eating more whole foods, which take away a lot of these questions. That said, you’ll still get trace amounts of 2-acetyl pyrazine from roasted or baked favorites, even if you bake everything from scratch. Safety organizations in the U.S. and Europe track new research. If risks ever change, regulations will likely follow.
People who want fewer additives should mostly shop the outer edges of the grocery store—produce, meat, dairy, whole grains. Specialty flavors play a small role in most diets. Right now, with studies suggesting current food-use levels won’t create harm, the concern isn’t high. Food should be about flavor and comfort, not a source of stress. Knowledge and moderation go a long way.
Open a bag of roasted nuts. Breathe in the scent of a bakery early in the morning. That deep, almost nutty smell weaving its way through bread, cereal, or chocolate likely owes something to a small but mighty molecule: 2-Acetyl Pyrazine.
I remember my first shift in a bakery, shoveling buns from the oven—what set the perfect loaf apart wasn’t only the texture, but the way it tempted your nose with this inviting, toasty aroma. Years later, in a flavor lab, 2-Acetyl Pyrazine came up all the time. It's a molecule that paints the rustic warmth that makes grains and snacks feel like comfort food.
Sniff a freshly popped bowl of popcorn and you immediately catch the signature of 2-Acetyl Pyrazine. It’s bold, nutty, a bit earthy. I’ve poured it onto sensory strips and found its smell lingering for hours—a sign of aroma staying power. You might also pick up subtle cracker, wheat, and toasted notes—the same comforting whispers found in baked products or brown rice.
Those who love coffee or roasted seeds know this experience too—the edges of the smell dance between cornflakes, peanut skin, and a touch of browned sugar. It brings unity to baked goods and snacks, which is why food companies turn to it when their products seem to be missing “that something.”
Once, while working on a cereal project, we found the base notes felt thin and artificial. A minuscule dose of 2-Acetyl Pyrazine changed everything. On the palate, it brings out roasted and nutty qualities much like what you taste in honey grahams or toasted sunflower seeds. Even a dash over the threshold draws out heartier, grainier, richer dimensions—the exact feeling you get from the crust of artisan bread.
There’s a fine line, though. Push the concentration too far, and the flavor grows overly earthy, almost woody, to the point it dominates rather than complements. Getting the dose right means the difference between barely-there background and intrusive off-note.
Consumers chase authenticity. Nothing gets folks suspicious faster than a product labeled “natural” that tastes hollow and lifeless. Ingredients like 2-Acetyl Pyrazine help manufacturers rebuild wholesome, appealing flavor after baking, extrusion, or other heat treatments have stripped away the soul. Researchers at the Monell Chemical Senses Center found that humans are sensitive to even faint traces—suggesting our bodies are primed from evolution for these roasted signals of nourishment.
There’s also the question of safety. Regulatory authorities consider 2-Acetyl Pyrazine safe in the low amounts found in foods, with decades of food use and research behind it. Still, transparency about ingredients remains essential. Older consumers, parents, and food professionals tend to value clear labels and responsible formulation.
Not every flavor challenge needs a chemical solution. Starting with high-quality grains, peanut blends, and mindful roasting carries more impact than masking shortcomings. But for food manufacturers facing shelf-life, cost, and consistency pressures, thoughtful use of 2-Acetyl Pyrazine can lift a flat recipe. This isn’t about shortcutting; it’s about finding ways to bring back some of the magic lost along the way.
Through years of tasting experiments and collaboration across food science, I’ve learned this: ingredients that build on what nature already delivers tend to win over the long haul. 2-Acetyl Pyrazine, used wisely, helps preserves those simple sensual joys of fresh, warm, roasted flavors—without having to compromise on enjoyment or safety.
Walking into a coffee shop or bakery, it’s easy to overlook the science that shapes our favorite tastes. 2-Acetyl Pyrazine plays a big role in that. This compound smells like popcorn or roasted peanuts, so food companies use it to dial up the warm, nutty notes in snacks. Potato chips, roasted nuts, even some chocolate bars benefit from a dash of it. During my first job at a family-owned food factory, we’d taste test new recipes. I remember how a tiny pinch of this compound could turn a dull cracker into something that tasted fresh out of the oven, full of those toasty, mouthwatering flavors. Food scientists rely on that consistency when they want to deliver familiar flavors, no matter the brand or type of packaged food.
Bakers love the compound for similar reasons. Sweet pastry fillings and crusts take on a richer profile with the help of 2-Acetyl Pyrazine. Croissants and puff pastries—especially those found on grocery store shelves—often contain this flavor enhancer to mimic that coveted bakery taste, even after weeks on the shelf. In dairy, especially cheese and cream-based products, it can highlight the browned, cooked milk notes people associate with homemade dishes. Many powdered cheeses and dairy sauces you find in convenience foods owe their roasted undertones to it.
Pets are picky in their own way. Anyone who’s shopped for fancy pet treats will recognize some of the same nutty flavors. Manufacturers use 2-Acetyl Pyrazine in kibble and treats to make them more tempting—after all, pet owners want their dogs and cats to be just as enthusiastic about mealtime as humans are. It helps to replicate the roasted meat aroma that triggers an instinctive, positive reaction in animals.
I grew up in a town with a cigar factory, and the blend masters I talked to always obsessed over subtle flavor notes. Cigarette and cigar makers use this compound to add depth to the tobacco flavor, so each puff delivers a hint of roasted, nutty richness. This chemical helps stabilize flavor differences in batches and gives products a reliable signature. Users experience a smoother, rounder flavor, making it valuable for both premium and mass-market producers.
Fragrance chemists reach for 2-Acetyl Pyrazine for its comforting, edible scent. Those cookies-and-cream candles or “baked bread” room sprays? They often rely on this compound to create a sense of warmth and welcome. There’s a reason so many air fresheners for homes and cars stick to bakery or nutty profiles—people associate these smells with safety and good times.
Some consumers worry about synthetic additives in processed foods, focusing on natural ingredients over anything created in a lab. But research has backed up the safety of 2-Acetyl Pyrazine at the levels used in foods and flavors. Regulators in the US and Europe keep a pretty close eye on exposure, and industry groups encourage manufacturers to be transparent about what’s inside a product. Investing in clear labeling and offering whole-food alternatives in product lines help brands serve both adventurous and cautious shoppers.
2-Acetyl Pyrazine makes food, fragrances, and even pet products more appealing. It offers a hint of comforting, roasted flavor, bringing familiar tastes and smells into everyday life, from a handful of popcorn to a slice of cheese toast.
Many people in labs might see 2-Acetyl Pyrazine as just another bottle on the shelf, but ignoring its storage needs can ruin its usefulness fast. Over the years, I’ve seen more than one batch spoil or turn yellow because someone tossed it by a sunny window or let it sit open in a humid storeroom. Bad storage habits don’t just waste money—they put results at risk, especially when every test run depends on chemical purity.
2-Acetyl Pyrazine brings that classic roasted, nutty aroma to the world of food chemistry. Because this fine substance is relatively stable, some folks might think it's tough and forgiving. But air and moisture in the room start nibbling away at it right from the first opening. Even trace contamination can dull its scent or set off unwanted reactions.
Room temperature—about 20 to 25°C (68 to 77°F)—works fine, as long as the area avoids temperature swings. Lab freezers aren't necessary, but it's smart to steer clear of heat sources and direct sunlight. Stray light breaks down pyrazine compounds slowly but surely. Even the best batch loses its punch sitting out on display under fluorescent bulbs.
Bottle caps deserve just as much attention. Exposure to air triggers slow oxidation and pulls in water vapor from the atmosphere. Once moisture creeps in, crystals can form or the whole solution may go cloudy. A tightly sealed amber glass bottle does the job best. Amber glass cuts out UV exposure, while a snug cap keeps out air and water. Think of storing fine coffee or spices—it’s the same idea: keep the good stuff sealed up and cool.
Many chemical storerooms get crowded. Stack flammable bottles together, and you set up conditions for accidents or degraded chemicals. 2-Acetyl Pyrazine won’t explode if ignored, but poor storage hurts its quality. Always label bottles with the opening date and watch for weird smells, leaks, or color changes. If anything seems off, don’t take chances—replace it.
Don’t store with strong oxidizers, acids, or bases. Contact with reactive chemicals degrades 2-Acetyl Pyrazine or could even create unexpected byproducts. Keep it in its own section with other stable aromatics and not near strong-smelling stuff like phenol or solvents that could mess up aroma-sensitive work.
Instituting regular checks of chemical storage isn’t busywork. Picking up the habit of labeling, sealing, and keeping containers clean pays off each season. Many labs overlook environmental logging, but recording room temperature and humidity helps spot issues before they turn into losses. Training new staff matters more than fancy storage fixtures. Discussing the impact of light, air, and cross-contamination sticks with technicians long after their first week. It’s about being mindful, not paranoid.
Researchers and technicians working with 2-Acetyl Pyrazine should treat it a bit like a fine ingredient in a chef’s pantry. Respect for packaging, clean hands, and the discipline to return it to a dark, dry spot preserve both quality and safety. The best science, after all, relies on reliable materials—and some lightly faded bottle tucked in a warm, sunny window doesn’t cut it.
| Names | |
| Preferred IUPAC name | 1-(pyrazin-2-yl)ethan-1-one |
| Other names |
2-Acetylpyrazine 2-Acetyl-1H-pyrazine Methylpyrazinyl Ketone 1-Pyrazinyl Methyl Ketone 2-Pyrazinyl Methyl Ketone |
| Pronunciation | /tuː əˈsiːtɪl paɪˈreɪziːn/ |
| Identifiers | |
| CAS Number | 22047-25-2 |
| Beilstein Reference | 1208734 |
| ChEBI | CHEBI:51511 |
| ChEMBL | CHEMBL182720 |
| ChemSpider | 6345 |
| DrugBank | DB04257 |
| ECHA InfoCard | 210-946-7 |
| EC Number | 2.3.1.20 |
| Gmelin Reference | 62239 |
| KEGG | C06172 |
| MeSH | D010357 |
| PubChem CID | 12568 |
| RTECS number | UY4375000 |
| UNII | 766C62NGU5 |
| UN number | UN3224 |
| Properties | |
| Chemical formula | C6H6N2O |
| Molar mass | Molar mass of 2-Acetyl Pyrazine: "122.14 g/mol |
| Appearance | Off-white to pale yellow crystalline powder |
| Odor | Nutty, popcorn, corn, roasted |
| Density | 1.105 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 0.07 |
| Vapor pressure | 0.00164 mmHg at 25°C |
| Acidity (pKa) | pKa = 12.5 |
| Basicity (pKb) | pKb = 11.92 |
| Magnetic susceptibility (χ) | Magnetic susceptibility (χ): -59.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.551 |
| Viscosity | Viscosity: 1.24 mPa·s (25 °C) |
| Dipole moment | 2.48 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 186.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -107.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2894 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | Precautionary statements: "P261, P264, P271, P272, P273, P280, P285, P301+P312, P302+P352, P304+P340, P304+P341, P305+P351+P338, P312, P322, P330, P332+P313, P333+P313, P337+P313, P342+P311, P362, P363, P403+P233, P501 |
| NFPA 704 (fire diamond) | 2 1 0 |
| Flash point | 61°C |
| Autoignition temperature | 410°C (770°F) |
| Explosive limits | Lower: 1.8%, Upper: 11.5% |
| Lethal dose or concentration | LD50 (oral, rat): 1,430 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat 1800 mg/kg |
| NIOSH | TT4250000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.05% |
| Related compounds | |
| Related compounds |
2,3,5,6-Tetramethylpyrazine 2-Methylpyrazine 2-Ethylpyrazine 2-Acetyl-3-methylpyrazine Trimethylpyrazine |