Back in the days before streamlined organic synthesis, discovering new molecules often meant plenty of trial, error, and late nights. Chemists stumbled onto derivatives like 2-Acetyl-5-Bromothiophene while searching for tweaks to the original thiophene skeleton. The journey to this compound started with humble experiments, substituting atoms and adding acetyl groups to see how the molecule would behave. Researchers in the mid-20th century became increasingly interested in halogenated thiophenes after early patents emerged on new dye intermediates and pharmaceutical precursors. The introduction of the bromo atom at the fifth position reflected a growing trend: pushing molecular diversity to unlock fresh properties for modern needs.
2-Acetyl-5-Bromothiophene, or 5-Bromo-2-acetylthiophene as you’ll see in catalogs, stands out in research circles for its direct path to more complex molecules. The structure's bromo handle and carbonyl group aren’t just decorations — they turn the ring into a hotspot for further reactions that feed straight into the world of pharmaceuticals, agrochemicals, and material science. You come across this compound honestly in the middle ground between basic feedstock and high-value specialty chemical, too rare for retail shelves but familiar among custom synthesis firms and university labs. Most chemists see it less as a finished product and more as a springboard.
You’ll spot 2-Acetyl-5-Bromothiophene in its off-yellow to pale brown crystalline form, a sign of its sturdy aromatic core. The melting point commonly sits in the 45–48°C range, which hints at a solid that handles gentle warmth but starts to liquefy in a busy lab. Its moderate molecular weight, around 219.07 g/mol, reflects the combined punch of sulfur, bromine, and the acetyl group. The compound dissolves decently in standard solvents like acetone and chloroform and resists easy break down in water, making it practical yet manageable when handling extractions and purifications.
Labels for research-grade 2-Acetyl-5-Bromothiophene usually emphasize the chemical formula C6H5BrOS and highlight batch purity levels: 97% and upwards in well-stocked shelves. Reputable suppliers tag samples with CAS number 13694-37-2, which settles confusion from overlapping synonyms. Other numbers hit labels as well: boiling point near 273°C, density hovering around 1.7 g/cm³, and typical storage instructions lean on dark, cool, and dry, a familiar refrain in synthetic labs. Detailed safety data sheets assure technicians of hazard statements and best storage practices right next to the compound name.
The standard synthesis moves through halogenation of 2-acetylthiophene with brominating agents like N-bromosuccinimide (NBS), shaping the molecule with predictable outcomes, provided the reaction temperature sits under control and the solvent choice guides selectivity. Skilled chemists aim for directness—less byproduct, more yield. The acetyl group gives enough latitude to tinker with electron density during bromination, which can up the final yield over brute force methods. This approach keeps waste down and cuts out unnecessary purification steps, earning a spot in efficient bench-scale syntheses without resorting to tricky or dangerous intermediates.
With both a reactive acetyl and bromine on the ring, chemists find all sorts of creative springboards. Cross-coupling reactions leap to mind. The bromine becomes the perfect leaving group for Suzuki, Heck, or Stille reactions, opening the door to a library of 2-acetyl-substituted thiophenes. You can swap out the acetyl group through reductions or enhance its complexity by simple condensation with amines, hydrazines, or acid chlorides. The sulfur atom, forever eager to participate in ring transformations, adds more utility, allowing further functionalization or ring expansion into more complex heterocycles used across pharmaceuticals and advanced materials.
The world of chemical catalogs and research articles tosses out plenty of names for this compound: 2-Acetyl-5-Bromothiophene, 5-Bromo-2-acetylthiophene, and even 1-(5-Bromothiophen-2-yl)ethanone, though this last one makes the systematic chemists happiest. Write-ups sometimes ditch the hyphens, but no matter how the name bends, it’s the same compound at its core. Even product numbers from different chemical suppliers circle back to the same molecular backbone, with registry like 13694-37-2 keeping the bookkeeping straight.
Working with halogenated aromatics like this one demands more than goggles and fume hoods; real diligence comes from understanding what you’re handling. Short-term exposure to dust or vapor can irritate the eyes and airways, the usual for electrophilic bromine compounds. Standard operating procedures flag the need for chemical-resistant gloves and splash-proof coats, but experienced chemists always go one step further, keeping spill kits on hand and cleanup routines tightened up. Disposal means collecting waste in halogenated organics streams, away from sink drains, and adhering close to institutional safety guidelines. Storage asks for tight, amber glass containers tucked away from sunlight and separated from oxidants—nothing gets left to chance where laboratory safety is concerned.
Pharmaceutical synthesis likes starting materials that punch above their weight, and this molecule regularly steps up as a precursor for anti-inflammatory, anti-infective, and antifungal drug candidates. The ability to shuffle its substituents across the thiophene core lets medicinal chemists probe new biological targets without tediously rebuilding molecules from scratch each time. Outside pharma, 2-Acetyl-5-Bromothiophene finds a home in organic electronics, where functionalized thiophenes anchor new semiconductors and photovoltaic materials. This compound lays the groundwork for new light-emitting devices and conductive polymers made to shuffle electrons efficiently. Agrochemical research draws from this molecule too, creating insecticides and herbicide leads that bridge traditional organics with tough sulfur-containing scaffolds.
At the cutting edge, research teams lean into structure-activity relationship studies, tweaking 2-Acetyl-5-Bromothiophene to dig for new drug candidates with improved potency and safety. Laboratories investigating new organic semiconductors keep testing fresh modifications, adding bulk or tweaking electron density around the ring, chasing the next leap in conductivity or stability. Experimental protocols often document yields, side products, and physical parameters in exhaustive detail—each dataset quietly building a public understanding of what this compound can and can’t do. As patent filings keep cropping up for substituted thiophenes, industry R&D groups keep refining synthetic efficiency and green chemistry credentials, aiming for cleaner, higher-yielding preparation strategies.
Toxicological studies work their way through the potential hazards, dosing cell cultures and small model organisms with compound solutions to nail down acute and chronic toxicity. Most reports mark 2-Acetyl-5-Bromothiophene as a mild skin and respiratory irritant, with higher concentrations prompting warnings about neurotoxic and hepatotoxic effects in poorly ventilated settings. Research still chases comprehensive data on environmental persistence and biodegradability since halogenated aromatics can resist breakdown, building up in soils and water sources over time. Progressive labs actively develop cleanup strategies and advanced filtration for spent solvents, trying to put a lid on environmental load before regulation tightens even further.
Forward-looking chemists believe 2-Acetyl-5-Bromothiophene's real strength comes from its barely tapped versatility. Ongoing studies in material science plot new applications in printable electronics, flexible displays, and bioactive coatings. As regulatory bodies begin tightening standards around solvent use and chemical waste, companies designing cleaner, lower-waste synthetic methods are likely to make survival easier for specialty intermediates like this. Attention to environmental health, safer reaction conditions, and smarter waste disposal should guide future work on the compound. This blend of practical chemistry and forward-thinking runs through each new application, quietly shaping how both industry and academia use and think about molecules like 2-Acetyl-5-Bromothiophene.
Chemical purity trips up plenty of people outside the lab. 2-Acetyl-5-Bromothiophene looks like a mouthful, but it’s just one specialty building block for pharmaceuticals or advanced materials. Sitting at the intersection of chemicals and everyday use, what matters most is how clean this compound really is. Purity often decides final product performance, especially in fields swinging between tight regulations and high expectations.
For research and many types of synthesis, chemists go after 2-Acetyl-5-Bromothiophene with purity notched at least at 97%. Most suppliers openly list this value. Take Sigma-Aldrich or TCI—they offer the compound with a minimum 97% chemical purity. That tells you, for every 100 grams, just three grams might stray off as unintended byproducts or trace water. In pharma or electronics, tasks may nudge that number even higher, because even a sliver of impurity detours results.
Many manufacturers sweat over contamination for simple reasons: patent protection, product safety, and stable yields. Impurities in 2-Acetyl-5-Bromothiophene don’t just spell problems on paper—they introduce unpredictable side reactions. A new antihypertensive medicine, made with a dirty batch, could end up causing more harm than good. In my time shadowing a chemist, watching them run compound after compound through thin-layer chromatography taught me that even point-five percent dirt can turn a smooth reaction into a nightmare. The tiniest hint of impurity makes scale-up risky and piles up unnecessary costs.
Beyond the bench, downstream users want fast, reliable validation for each bottle. Let’s say someone’s building a next-gen organic transistor—unwanted molecules interfere with electrical response, wasting research dollars and time. A university project I was involved in saw a fortnight lost tracing an electrical hiccup back to a poorly sourced intermediate.
Purity sounds simple, but reaching those high marks often wrestles with costs and lab resources. Making 2-Acetyl-5-Bromothiophene sparkle above 98% means more than just running it through a machine—it’s stepwise distillation, pricey resins, careful temperature controls. Sellers aiming for mass production sometimes sacrifice purity just to keep spreadsheets happy. Rushed orders and poorly stored stock can tank purity further. Once, chasing after a time-sensitive synthesis, we found brownish specks clinging to a sample, only to later discover the supplier kept their reagent freezer on the fritz for weeks.
Fact-checking a product’s certificate of analysis matters more than trusting a catalog description. Reliable sellers show their HPLC or NMR spectrograms, instead of just quoting a single number. Labs, if resourceful, run a quick in-house purity check before diving into a batch reaction, even if the supplier has a golden reputation. Better funding for material control and a little extra scrutiny save headaches later—and only a handful of dollars compared to troubleshooting a failed project. If more folks in research and industry pushed for transparency, labs and companies would feel less pressure to cut corners. Clearer regulations demanding complete breakdowns of impurity profiles, not just one percentage, would put more confidence in every shipment.
Anytime researchers talk about building blocks for medicine, there’s a good chance thiophene rings show up. 2-Acetyl-5-Bromothiophene steps into drug labs as a solid starting point for chemists hunting new molecules. With its bromine and acetyl groups, it breaks into new territory when creating bigger, more complex scaffolds, especially for compounds with strong biological activity. Modern synthesis keeps looking for shortcuts, and this compound offers a shortcut—both a handle for precise chemical changes and a steady base for adding further pieces.
Examples show up: Some anti-inflammatory agents, certain antibiotics, and anti-cancer lead structures begin with thiophene derivatives like this one. Drug discovery doesn’t rely on one approach, but the search for selective therapies puts compounds like 2-Acetyl-5-Bromothiophene under the spotlight for trial-and-error exploration.
Agriculture faces plenty of challenges, from fighting crop diseases to boosting yields in unpredictable weather. Chemical companies have long blended thiophene rings into new pesticides and herbicides. 2-Acetyl-5-Bromothiophene, with its structure, gives chemists a way to branch into unexplored candidates. You see this molecule in the early screening stages. Researchers use it when tweaking and testing for the right mix of activity and safety—both to help crops and avoid unnecessary environmental harm.
Experience says, discovering pest controls isn’t just about power. There’s a focus on selective targeting, which keeps beneficial insects out of harm’s way. Laboratory runs often start with base molecules like this one, then get reshaped with small changes that lead to big differences out in the field.
Material scientists also recognize 2-Acetyl-5-Bromothiophene as a useful piece in the design of organic functional materials. Organic electronics, for example, keep grabbing headlines—conductive polymers for solar cells and OLED displays owe a lot to thiophene chemistry. Adding a bromine at just the right place allows careful linking with other molecular fragments, changing properties like electrical conductivity or making thin films respond to light in different ways.
In my own dabbling with organic synthesis, swapping functional groups on thiophenes often turned idle curiosity into working prototypes. These building blocks shape themselves into compounds that conduct electricity or display new colors, so broadening their reach extends the tools on hand for designing better screens, sensors, or light-absorbing devices.
People outside the lab sometimes forget how much material innovation depends on chemical building blocks. A single new derivative can shift the performance of a whole product line, trimming costs or ramping up durability.
Growing demand for tailored chemicals in medicine, food, and tech keeps the search for new building blocks alive. While much of the work stays hidden in corporate or academic labs, those on the outside will see the results in safer crops, smarter gadgets, and better access to medicines. If the chemical industry can keep the supply of building blocks like 2-Acetyl-5-Bromothiophene steady, and push for cleaner ways to make and use them, there’s room to solve more problems without causing new ones.
Chemistry pushes modern life forward. Each time a new scaffold gets built, the odds of stumbling on something useful go up. In the case of compounds like 2-Acetyl-5-Bromothiophene, their steady demand proves how small changes in basic molecules unlock new opportunities—sometimes in areas we weren’t even looking for.
Every time I walk past the chemical storage in a lab, I think about how easy it is to overlook the basics. Folks often see a shelf lined with brown glass bottles as just another part of the job, but those tiny details shape how well our experiments go and whether everyone stays safe. Take 2-Acetyl-5-Bromothiophene. It’s not the household name that acetone or bleach is, but for chemists, it’s part of a toolkit that builds drugs and advanced materials. The catch: it doesn’t last forever, especially if you stash it carelessly.
Heat spins many chemicals out of control. My earliest job involved tagging the thermometer daily in a storage closet, and it stuck with me how even mild temperature swings could turn a good sample into a disaster. For 2-Acetyl-5-Bromothiophene, room temperature works well, but no one wins by letting it roast near a radiator or in the sun. Steady, cool conditions help. Keeping it somewhere between 20°C and 25°C stops the breakdown game before it even starts.
Bright light also sets off slow changes. I watched someone once pour a similar compound near a window, and after weeks, the color looked off. Sunlight, even the indirect kind, can nudge sensitive chemicals into misbehaving. Tucking bottles away in cabinets or behind tinted glass takes just a minute. Good labs use brown bottles because they act like sunglasses for the liquids inside.
In my years of chasing down “why did this sample go bad?” stories, humidity pops up often. Air brings in water vapor, and for compounds like this, that’s a problem. Water can gum up the chemistry. Keep lids tight: screw caps do the trick, and a strip of parafilm adds a cheap extra barrier. I’ve seen busy benches where bottles get left half-opened — that’s the classic start to contamination or spoilage.
Oxygen also sneaks in and, given enough time, will change the personality of the molecule. My routine always included double-checking that everything was properly closed and that nothing sat exposed longer than it needed to. This everyday diligence pays off, because replacing spoiled chemicals isn’t just annoying; it eats into budgets and project deadlines.
Details matter here. Fresh out of college, I didn’t grasp why clear, dated labels were such a big deal, but I got it quickly after seeing a batch get tossed because the shelf life was anyone’s guess. Good labeling means no mysteries: write down the purchase and open dates, and check material safety sheets for recommended expiration. Rotating stock so the oldest gets used first is more than habit — it lowers waste and risk.
I’ve heard stories of accidents tied directly to old, poorly kept chemicals. Pieces fall into place after the fact: leaky seals, faded labels, bottles that got too warm on forgotten shelves. One can prevent most of these headaches with a simple checklist and a dose of respect for what’s on those shelves. It pays to be the person in the lab who pushes for clear policies and steady habits.
A lockable, dry cabinet, cool and away from direct light, checks nearly all boxes. Simple shelf dividers help avoid confusion between similar bottles. Training new folks on basic storage routines feels tedious, but it’s how you dodge trouble in the long run. For chemicals with specific hazards, always check if a regular fridge or a spark-proof model is needed; standard kitchen fridges sometimes invite trouble with flammable or volatile compounds.
Smart storage doesn’t belong on a “maybe” list — it’s a team effort that keeps labs safer, saves money, and helps experiments succeed the first time. Years of working around creative scientists have convinced me: a bit of practical care with storage turns up better results than any clever shortcut down the line.
Bottles filled with unfamiliar names can intimidate anyone new to experimental chemistry. I used to work in a small lab where new reagents seemed to arrive every week, and 2-Acetyl-5-Bromothiophene once caught my eye. A thiophene ring, an acetyl group hanging from one side, and bromine tucked at another spot—this isn’t something you find at the grocery store. Given its structure, several concerns pop up in the back of my mind, built from years of mixing, heating, and cleaning up all types of organic chemicals.
2-Acetyl-5-Bromothiophene comes straight from the world of chemical synthesis. It sees use as a building block in pharmaceutical research and sometimes in the flavor and fragrance industry. Structurally, thiophene brings a whiff of sulfur; bromine offers reactivity, and the acetyl group means potential volatility. Each piece of the puzzle raises flags, especially compared to simple solvents or salts. When chemicals combine aromatic rings, sulfur, bromine, and active groups, they bring the real chance to irritate, harm organs, or demand extra caution.
Any compound with a halogen (bromine in this case) usually makes gloves and good ventilation critical. Organic bromides can be tricky, sometimes producing nasty gases in the wrong reaction. The acetyl part brings volatility—a faint, sweet smell that means it might evaporate and sneak through weak respirators or cause headaches. No one wants to breathe something they can’t pronounce, and many acetylated compounds can irritate the nose, throat, or eyes after short exposure. The researchers I’ve worked alongside always treated such chemicals like the quiet students who never speak up in class—never causing problems until the one day you forget to show respect.
Splash this stuff on skin and you’ll likely notice burning or redness. Get it in your eyes and you’ll feel pain and cloudiness. So, having chemical splash goggles and nitrile gloves happens for a good reason. More than one chemist I know made the mistake of using latex gloves—the wrong choice since many solvents and some brominated chemicals slip right through. Ventilation keeps headache and nausea away as fumes accumulate without fans or fume hoods. Getting lazy cleaning up spills invites long-term problems; these aren’t just stains, but often signals the absorbed chemical may end up in the skin or air later on.
Planning beats panic. Storing 2-Acetyl-5-Bromothiophene in a chemical refrigerator (not the lunch fridge) cuts down accidental inhalation. Solid procedures matter above all: goggles become comfortable with time, gloves never last past the reaction, and waste gets labeled and locked away each time. Emergency washes and fire blankets must stay visible, not shoved in a corner. Training systems in labs work best when people actually use them—complacency brings bad luck. Respect for the chemical means checking SDS sheets, reading fresh labels, and reminding those around me why shortcuts tempt, but never save time in the end.
Small labs and big companies both feel the cost of careless mistakes. 2-Acetyl-5-Bromothiophene isn’t out to hurt anybody, but without the right steps, it will. By remembering hard-won lessons and sticking to clear safety routines, chemists leave less to fate and more to careful hands. That’s how more people make it from one experiment to the next with stories—rather than scars—to share.
2-Acetyl-5-Bromothiophene might sound complicated, but organic chemistry throws names like this around all the time. For someone who has juggled glass beakers and balanced endless chemical equations at the lab bench, these long names start making sense once you break them down. The name even clues you in on how the molecule is put together.
Take the chemical formula: C6H5BrOS. This isn’t a pattern of letters people memorize for fun. Each symbol and subscript tells a story about what elements are present and how many atoms of each are working together. In the case of 2-Acetyl-5-Bromothiophene, there’s a benzene ring at its base, with substitutions that set it apart in structure and behavior from other similar compounds.
Plenty of research labs focus on thiophene rings because these structures pop up in areas ranging from pharmaceuticals to OLED displays. So, knowing exactly how many atoms, and which types, creates a common language for scientists in countries across the world. A single mistake in counting the atoms can send an entire project off the rails. Students learn this lesson the hard way in the first organic chemistry class, losing points for a misplaced number, but scale that up to a pharmaceutical company synthesizing this compound for a drug candidate and the problem grows much larger.
The molecular weight for 2-Acetyl-5-Bromothiophene clocks in at 219.08 g/mol. Anyone who’s measured out a compound in a fume hood knows the value of this number. Even one decimal off could throw off dose calculations and chemical yields. Imagine making a batch of a painkiller or an antibiotic, only to realize the initial formula was off by a fraction. The wrong weight can lead to waste or, far worse, ineffective or dangerous medicines.
I remember, as a young chemist, using dusty electronic balances and calculating weights painstakingly on paper. Slip once and the experiment fails, costing hours, or even days, in a research cycle. In manufacturing, the stakes are higher can make or break production lines.
Chemical reactions thrive on precision. Each element in the formula plays a part. Bromine sits as a heavy atom, sulfur links to the aromatic ring, and oxygen pulls into the acetyl group, adding polarity and opening doors to more reactions. Chemists who get to know these details can predict reactivity, think up potential uses, and fine-tune reactions.
For companies making advanced electronics, a single molecule’s weight might change how thin films build up, or how light passes through a screen. Those who care about new drugs or sustainable materials need this level of detail. Mistakes in the basics ripple outward, turning into costly recalls or lawsuits, or products that never leave the prototype stage.
There’s still work to do in teaching this foundation. Many undergrads jump to memorizing names without building a habit of checking the math behind them. Better lab practices, double-checking results, and using smarter lab management software can go a long way toward catching mistakes. Open databases and collaboration between research groups help, too. Letting scientists across the planet spot-check chemical data keeps errors from snowballing and ups the reliability of innovation in the process.
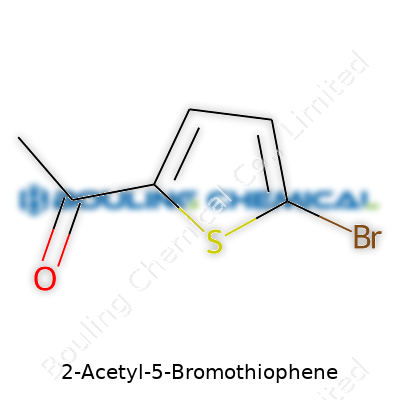
| Names | |
| Preferred IUPAC name | 1-(5-bromothiophen-2-yl)ethan-1-one |
| Other names |
2-Acetyl-5-bromothiophene 1-(5-Bromothiophen-2-yl)ethan-1-one 5-Bromo-2-acetylthiophene 5-Bromo-2-thienyl methyl ketone |
| Pronunciation | /tuː-əˈsiːtɪl-faɪv-ˈbroʊmoʊ-θaɪˈoʊfiːn/ |
| Identifiers | |
| CAS Number | [Pick one] "13632-98-7 |
| 3D model (JSmol) | `3D model (JSmol)` string for **2-Acetyl-5-Bromothiophene**: ``` CC(=O)c1ccc(Br)s1 ``` |
| Beilstein Reference | 1718983 |
| ChEBI | CHEBI:189823 |
| ChEMBL | CHEMBL136869 |
| ChemSpider | 177860 |
| DrugBank | DB07723 |
| ECHA InfoCard | 100.031.229 |
| EC Number | 218-726-7 |
| Gmelin Reference | 8984 |
| KEGG | C19122 |
| MeSH | D017670 |
| PubChem CID | 120586 |
| RTECS number | KL8400000 |
| UNII | 7U4M5019D7 |
| UN number | UN2810 |
| Properties | |
| Chemical formula | C6H5BrOS |
| Molar mass | 231.08 g/mol |
| Appearance | White to off-white solid |
| Odor | strong, sweet, popcorn-like |
| Density | 1.759 g/cm³ |
| Solubility in water | Insoluble in water |
| log P | 1.9 |
| Vapor pressure | 1.13E-3 mmHg at 25°C |
| Acidity (pKa) | 6.93 |
| Basicity (pKb) | -4.7 |
| Magnetic susceptibility (χ) | -61.94·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.6030 |
| Viscosity | Viscous liquid |
| Dipole moment | 2.91 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 267.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -30.9 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | –3377.8 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P280, P304+P340, P312 |
| Flash point | 68°C |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 >2000 mg/kg |
| NIOSH | SN1225000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 2-Acetyl-5-Bromothiophene: Not established |
| REL (Recommended) | 10 mg |
| Related compounds | |
| Related compounds |
Thiophene 2-Acetylthiophene 2-Bromothiophene 5-Bromothiophene-2-carboxylic acid 2-Acetyl-4-bromothiophene |