Decades ago, food chemists stumbled onto a family of molecules that could turn bland products into something people could actually crave. The search for flavor compounds led right to the door of pyrazines, with 2-Acetyl-3-Methyl Pyrazine drawing special attention. At the time, flavorists working with cocoa and coffee soon noticed pyrazines added that warm, roasted, nutty character. The molecule picked up steam in the synthetic flavors industry through the 1970s and 1980s. Innovation came not just from corporate flavor labs, but also from university kitchens trying to mimic natural aromas. Nowadays, practically every processed snack on the shelf owes its richness and moreish quality to the early focus on these little nitrogen rings.
Shoppers scanning labels usually won’t recognize the name. 2-Acetyl-3-Methyl Pyrazine hides in ingredient statements under “natural flavor” or “artificial flavor.” In practice, this compound’s warm and nutty aroma works wonders in baked goods, chocolates, cereals, and roasted snacks. Unlike vanilla or cinnamon, it adds punch—you get depth, toasted notes, hints of popcorn, even a suggestion of browned butter. Snack companies prize it for its low detection threshold; just a whiff and a biscuit transforms, offering that full-bodied toasty flavor you only find at the edge of caramelization.
Without introducing unnecessary jargon, this compound runs off a simple formula: C7H8N2O. It takes the form of a pale yellow oil at room temperature, with an unmistakably potent aroma even in tiny amounts. Solubility in alcohol and organic solvents makes it useful for flavoring both solid and liquid products, but it does not dissolve perfectly in water. Its boiling point hovers around 158 degrees Celsius at low pressure. Its scent profile stands out so clearly that trained chemists recognize it instantly in a GC-MS trace.
Industry needs clarity about purity, so typical commercial batches advertise a purity of at least 98%. Packages line up with food-grade labeling standards, listing batch number, date of manufacture, and storage recommendations. Manufacturers keep a close eye on residual solvents and heavy metals, keeping levels well below safety thresholds. Companies selling to flavor houses provide detailed COAs, aiming for transparency around identity and traceability, reflecting growing regulatory expectations and the trust consumers want when they buy a product with a complicated supply chain.
Most of the world’s supply comes from a reaction between 2,3-butanedione (diacetyl) and methylamine or other short-chain amines under controlled conditions. The classic synthesis uses thermal cyclization—a heated environment pushes these building blocks together to form that signature pyrazine ring. Reaction yields depend on factors such as temperature, catalyst presence, and timing, as side products build up if the mix overheats. Smaller producers stick to batch processes, but giants scale up using continuous reactors for efficiency, using modern controls to maintain high purity and catch impurities early.
2-Acetyl-3-Methyl Pyrazine doesn’t just stand alone. Chemists frequently tweak its structure to fine-tune flavor profiles. Adding longer alkyl groups or swapping in different side chains shifts the aroma, sometimes toward earthiness or even chocolate notes. Reaction with aldehydes or further alkylation can create a whole family of related compounds tailored to specific confectionery mixes. Many pyrazine derivatives arise not from synthetic labs but from the roasting process in actual foods, mirroring pathways used in food labs for new product formulations.
You won’t always see this compound by its chemical name. It turns up as 2-Acetyl-3-methylpyrazine, 3-Methyl-2-acetylpyrazine, or even under trade names tied to major flavor houses. Patent literature and supply catalogs swap between these variants, sometimes using code numbers that only insiders recognize. Regardless of the label, professionals reach for this molecule whenever they need to push a product into that next tier of sensory satisfaction.
Producers watch safety closely, as with any aromatic compound. Storage drums stay tightly sealed, away from heat and sunlight, to prevent degradation. Technicians handle the product in well-ventilated spaces; concentrated vapors can cause irritation. Industrial hygiene policies require gloves and eye protection. Update cycles for workplace training keep teams up to speed. Third-party audits and on-site sample testing make sure cross-contamination never becomes an issue for end users. Regulatory agencies, including the FDA and the European Food Safety Authority, list the compound for use in foods, but always urge reasonable exposure levels.
Bakeries and snack producers were some of the earliest adopters of 2-acetyl-3-methyl pyrazine. They add small quantities to cookies, crackers, popcorn, and cocoa-based goodies to deepen the roasted profile. Chocolate factories know that just a drop rounds out bitterness and creates a lingering finish. In some Asian markets, ramen seasoning and broths rely on pyrazines to lift umami impact. Tobacco blenders use it to replicate cured and aged leaf notes. Beverage companies draw on it for malt tones in beers and malted milk drinks. Even the pet food industry takes advantage of this molecule to make products more palatable to furry consumers.
Research continues to uncover new potential. Younger labs experiment with enzymatic or fermentation-based synthesis routes to cut down on chemical reagents. Sensory science teams apply high-resolution GC-Olfactometry to find just the right threshold for new snacks, measuring exactly how this molecule interacts with fats, salts, and sugars to achieve blockbuster flavors. Scientists in public research centers sometimes focus on the Maillard chemistry roadmap, tracing how heat, sugar, and amino acids spin out not only 2-acetyl-3-methyl pyrazine but a suite of alluring aroma compounds. Graduate students have published dissertations on sensory similarities between this pyrazine and certain aged spirits and roasted beans, proving that food science still has plenty of new territory to explore.
Decades of toxicology work support the use of this compound in food—within reason. Excessive intake just isn’t feasible due to the intensity of its aroma, so real-world exposures tend to remain safely below margins of concern. Animal studies set upper limits, and regulators periodically review available data to keep up with new findings. Some earlier concerns about volatile pyrazines rested more on theoretical risks, rather than documented harm. Today, labs keep busy running chronic exposure studies and checking for metabolic byproducts, staying out in front of the science so no surprise issues catch the food sector off guard.
Companies everywhere keep searching for better ways to unlock taste. As plant-based foods take off, demand for authentic roasted flavor only climbs. This puts pyrazines like 2-acetyl-3-methyl pyrazine in the spotlight, especially for vegan snacks and non-dairy sweets. There’s strong interest in producing these molecules from fermentation, either via bioengineered yeast or from spent grains, which could cut costs and appeal to sustainability-minded buyers. Regulations will continue adapting, pushing chemical firms to prove safety and provenance at every stage. As global populations grow and food choices diversify, look for this unassuming molecule to play an even bigger role in the food landscape, connecting everyday eaters to the comforts of roasted and toasted flavors.
2-Acetyl-3-Methyl Pyrazine is one of those ingredients most people have never heard of, but pretty much everyone has enjoyed. Toasted nuts, popcorn, roasted coffee, the smell of fresh-out-the-oven bread—this simple-sounding chemical gives these foods their signature aroma. Not in a flashy way. It’s that delicious, subtle “roasted” character working in the background.
Food companies lean on this ingredient all the time. It’s in things I grew up munching—breakfast cereal, flavored chips, nut butters, chocolate bars. It helps make processed foods taste like home cooking, even if it’s a shortcut. That “just-roasted” smell triggers nostalgia, and companies know people respond to that. Brands use it in everything from potato snacks to cocoa blends to amp up the comfort factor. The same goes for coffee flavorings. You find this molecule on the label of instant mixes and flavored creamers, adding back what’s lost in the big batch manufacturing process.
Restaurants and chefs love the edge it gives to seasoning blends. Sprinkle it in a soup and suddenly it tastes as if your veggies caramelized for hours. Bakeries sometimes add it to breads and cookies to get that golden-toasty scent people expect from baked goods. Even pet food companies use it so that kibble smells good enough for a dog owner to trust, which is smart business. The molecule is also in perfumery, turning up in “gourmand” scents—those perfumes that remind people of sweet, roasted treats.
I think most people don’t realize how much smells shape their experience of food and their willingness to try new things. Kids reach for what smells familiar. For some folks, medical conditions or age can dull their sense of taste, but scents like 2-Acetyl-3-Methyl Pyrazine’s roasted note still break through—making eating more enjoyable. There’s real comfort in it. One sniff, and it’s easier to connect with memories or with what’s on your plate.
Some people worry about anything sounding “chemical” in their food, but this compound’s found in nature—in roasted seeds, grains, nuts, coffee, and even baked potatoes. The food industry mostly makes it in a lab these days, because it’s efficient, reliable, and avoids stripping forests just for a tiny yield from natural sources. Honestly, synthetic doesn’t always mean bad. Quality and safety rules guide how much goes in foods, and most people eat tiny amounts at a time.
Food flavor trends move fast, but people keep coming back to what tastes familiar. For companies, adding 2-Acetyl-3-Methyl Pyrazine solves a taste challenge: how do you keep products consistently craveable, affordable, and safe? One fix—use powerful flavors where they count. Still, real kitchen skills and slow cooking bring their own rewards, and nothing from a bottle replaces that. As long as we know what’s in our food and why, we have a better shot at making smart choices for ourselves and our families.
Food companies love to use 2-Acetyl-3-Methyl Pyrazine. The name sounds intimidating, but this is the stuff that gives chocolate, roasted nuts, and some breads that warm, toasted aroma. In the kitchen, pyrazines form naturally during the roasting process. That nutty, almost popcorn-like scent in the air? That’s chemistry at work, not just nostalgia.
Big regulatory groups, like the FDA and the European Food Safety Authority, weighed in years ago. It landed on the list of food flavorings considered safe when used in the small amounts needed for foods and snacks. It’s pretty rare to see complaints or incidents linked directly to this compound.
The paperwork behind flavoring safety comes from animal tests and good old-fashioned math. Based on how much people typically eat, the exposure is hundreds or even thousands of times lower than the doses that cause problems in lab animals. Based on surveys and food diaries, most folks get only a few milligrams a year, not nearly enough to raise flags in the toxicology community.
I’ve worked in kitchens, tasted plenty of experimental foods, and known a few people on food ingredient teams. They all point out that 2-Acetyl-3-Methyl Pyrazine gets used at microscopic levels. A drop can change the flavor of a thousand bars of chocolate. Food scientists obsess over not only the taste but also the rules, because recalls are expensive and reputations matter.
I’ve noticed a lot of us get anxious reading ingredients we can’t spell. That makes sense given how little real discussion happens about what’s actually in processed goods. Yet, a quick scan through the evidence doesn’t turn up horror stories on this one. If I had to lay blame for health issues, I’d look at other things before tiny flavor molecules—think excess salt, sugar, or saturated fat.
Safety decisions don’t happen in a vacuum. A few years ago, some researchers raised questions about flavorings building up in the body or causing long-term effects. Most food flavorings—including this one—move through the digestive system without sticking around. The liver breaks them down, filters them out, and they leave the body in a day or two.
Still, not every risk gets caught right away. Overuse is the risk, not the flavoring itself. The food industry sometimes pushes flavor-forward processed snacks where it’s easy to wolf down half a bag. In that case, folks take in more sugar, salt, and calories—those add up a lot faster than a few molecules of this compound.
People still deserve to know what’s in their food. Labels don’t always spell out every single compound, which bugs a lot of careful eaters. More detailed labels would be a straightforward win. Knowing what’s inside lets shoppers steer clear if they want, without needing a chemistry degree.
Folks who worry about synthetic versus natural flavors may not realize that 2-Acetyl-3-Methyl Pyrazine pops up in roasted coffee, fresh bread, and home-cooked nuts, right alongside lab-made versions. The molecule doesn’t change because of the source—it’s about the structure, not the origin.
Curiosity helps. If people push for more transparency, companies have a reason to offer more info in plain language. Instead of hunting down every hard-to-pronounce ingredient, people could focus on eating a mix of foods, watching portions, and sticking mostly to items they actually cook. That approach really moves the needle on health, far more than the occasional trace of a toasted aroma compound.
2-Acetyl-3-Methyl Pyrazine, or 2AM, lands on a short list of ingredients that can turn plain food into something that smells and tastes just right. I’ve noticed how just a few drops of the stuff in a test kitchen can fill the room with that rich, roasted air that takes you straight to childhood breakfasts or sneaking a cracker from a busy stove. You get the sense that without these flavor enhancers, your favorite snacks would feel pretty forgettable.
Food technologists keep 2AM concentrations quite low. Most snacks, baked goods, cereals, and confectionery items contain 2AM rates from about 0.1 to 5 parts per million (ppm). People who have worked in food manufacturing know that even this tiny amount does the job. Companies use concentrations at the lower end for delicate dairy or grain flavors, and higher levels for bold, roasted snacks or some chocolate treats.
In seasoning blends and snack coatings, 2AM often sits around 0.2 to 1.5 ppm. Chocolate makers rarely go above 1 ppm, because too much brings off-flavors and an unnatural bite, which nobody wants in a comforting chocolate bar. In breakfast cereals, you’ll often see around 0.5–2 ppm just for a golden, baked note that stays in the background.
Anyone who’s tipped a beaker of this stuff into an experimental batch by mistake knows how the aroma bullies every other flavor. Lab notes are filled with reminders: sprinkle, never pour. Too much 2AM brings burnt, over-roasted, and bitter tastes that most people would push away. Companies play it safe both for flavor and for regulatory reasons—excess chemical flavorings can lead to legal headaches and product recalls. The FDA and other global agencies haven’t listed strict maximums for 2AM, but food chemists stay wary. They aim under the threshold where flavor turns into a complaint letter.
Manufacturers use highly concentrated forms—liquids or powders—that dissolve into large batches. Ingredient suppliers provide technical sheets, but it’s up to flavorists to tweak and taste until the batch feels just right. I’ve seen factories where the mixing teams are so cautious that they add the pyrazine in preblended seasoning packets, minimizing contact and mistakes. Small amounts—measured on a scale sensitive to milligrams—go into a ton of food.
The quest for vibrant flavors pushes companies to look for natural sources of similar compounds. Roasting grains or nuts, for example, generates 2AM and its relatives. Still, most food processors reach for the synthetic, since it is cheap, pure, and reliable.
Consumers keep asking questions about what’s in their food. If regulators tighten rules around added flavor chemicals, manufacturers might switch to more “natural” roasting methods or try fermentation tricks to nudge up these nutty, bready flavors. Some companies already experiment with special strains of yeast that produce pyrazines right in a dough. Technology will keep shifting, but the science says to keep flavors clear and quantities modest, because a bit too much ruins the snack.
2-Acetyl-3-Methyl Pyrazine packs a punch in the flavor world, especially in snacks and bakery products. It brings that freshly toasted note you find in roasted nuts or popcorn. Handling it isn’t all that complicated, but nobody should ignore the basics that keep a workplace safe and efficient. From my time around food labs and industrial kitchens, one thing’s certain: treat every liquid flavor with respect and you stay out of trouble and avoid unexpected expenses.
Most flavor compounds act pretty sensitive to the environment, and this one’s no exception. High temperatures chip away at its aroma and open the door to off-notes or color changes. I’ve seen what storing flavor chemicals near ovens or even in direct sunlight can do; it’s a fast track to waste and unhappy customers. Put this pyrazine somewhere dry and cool, and keep it out of those sunbeams. A shelf in a climate-controlled room or a dedicated chemical fridge beats the back of the storeroom every time.
2-Acetyl-3-Methyl Pyrazine gets along best with air-tight glass or high-quality plastic bottles. Screw caps or sealed containers make a real difference. Humidity reaches into every nook and cranny, spoiling the chemistry, so take that seriously. If a facility sits in a humid place—think subtropical warehouses—a dehumidifier buys peace of mind. Leaky or poorly closed lids end up costing far more than the time it takes to screw one on tightly.
Flavors in high concentration can get overwhelming fast. People often forget how intense the aroma can be, and a small spill lingers. Gloves and safety glasses are more than window dressing: they keep sticky situations from turning into actual hazards. I remember a coworker once getting a small splash on their hands, and the smell lasted for days, not to mention the mild skin irritation. It pays off to use the provided PPE and work in a well-ventilated spot.
Unmarked bottles lead to confusion, wasted material, and sometimes much worse. Good practice means clear, durable labels—product name, date of arrival, and any hazard warnings. In my own experience, clear labeling saves the day on inventory checks and keeps accidental mixing to a minimum. Rotating stock (using the older material first) cuts down on surprises at recipe scale-up or lab bench testing.
A minor spill rarely stays minor on a busy floor. Keeping absorbent towels or chemical spill kits within arm’s reach prevents a simple mishap from spreading across the workspace. Used materials must go straight into the correct waste stream; local disposal rules vary, but you should never pour anything like this into a sink or trash can. I’ve seen companies get hefty fines for poor waste handling, so it makes sense to check the protocol before anyone cleans up.
Some folks overlook batch dating and regular checks on stored chemicals. Regular audits—monthly or quarterly—help spot degraded stocks before they end up blending into production runs. I once found several bottles at the back of a shelf that had changed color due to age; they looked fine from the outside, but one whiff and it was clear they were past their prime.
Storing and handling flavors like 2-Acetyl-3-Methyl Pyrazine doesn't require fancy equipment, just consistent habits and a clear system. Protect the material from heat, moisture, and light. Cap every bottle tightly. Keep labels clear and waste disposal procedures sharp. These steps protect both the people and the investment, ensuring that the distinctive aroma never goes to waste.
Open a bag of toasted corn chips or sniff a freshly baked loaf of bread and there’s a good chance you’re catching a whiff of 2-Acetyl-3-Methyl Pyrazine. This flavor compound shows up often in food products because it packs a deep, roasted note with some nutty edges. Using it makes sense: it transforms bland cereal bases into bowls of comfort and helps snacks smell warm and inviting. But for anyone working around food safety, every additive brings its own list of questions—especially about allergens and regulatory controls.
Allergic reactions hit close to home for a lot of folks, including many in my own circle who scan ingredient decks for “hidden” risks. 2-Acetyl-3-Methyl Pyrazine doesn’t belong to the list of top allergens, which include milk, peanuts, tree nuts, and a handful of others. It isn’t derived from nuts, dairy, or shellfish, either. Most of this compound that goes into foods gets made synthetically, which means it doesn’t drag along plant or animal proteins known for triggering allergies.
Real-life examples back that up. Food safety agencies and research literature rarely — practically never — list reactions tied to this molecule. I’ve dug through European Food Safety Authority (EFSA) opinions and U.S. Food and Drug Administration (FDA) databases and didn’t find warnings singled out for this flavoring. It’s not say there’s zero risk—folks can react to almost anything in large enough amounts—but this isn’t an ingredient that pops up in allergy clinics or food allergen recalls.
Regulators keep a close eye on food additives. In the United States, the FDA tags 2-Acetyl-3-Methyl Pyrazine as “generally recognized as safe” (GRAS). It has a FEMA (Flavor and Extract Manufacturers Association) designation—number 3289, for the record. All this means labs have evaluated it, studies checked for acute (short-term) and chronic (long-term) toxicity, and no red flags forced it out of circulation.
Europe takes its own approach. EFSA reviewed this flavor along with a bunch of other pyrazines. They approved it under flavoring group 14.078, meaning foodmakers across the EU can use it according to good manufacturing practice. Japan’s Ministry of Health, Labour and Welfare lists it as an approved flavoring, too.
Companies still track their use. Additive limits keep amounts in finished products far below “no observed effect” thresholds. I’ve worked with teams that monitor total intake from all food sources—so totals never edge past what regulatory agencies set out as “safe.” Regulatory rules also push for labeling transparency, usually listing this molecule as a “flavoring” unless it’s present at high enough levels to require specific mention.
Even with a long safety history, food science isn’t a “set it and forget it” field. Keeping an eye on new data is part of the job. Manufacturers and importers keep in touch with agencies, ready to pivot if someone documents an unexpected reaction. Suggesting clearer labels—even for flavorings seen as safe—would help people with food sensitivities. Extra research into the effects of flavor complexes, not just single molecules, could reduce future risks by clarifying what happens in real-life diets instead of focusing only on compounds in isolation.
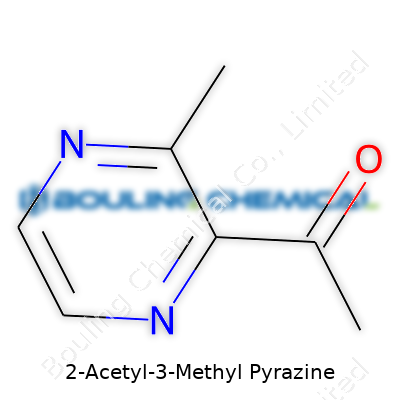
| Names | |
| Preferred IUPAC name | 1-(3-methylpyrazin-2-yl)ethan-1-one |
| Other names |
2-Acetyl-3-methylpyrazine 3-Methyl-2-acetylpyrazine 2-Acetyl-3-methyl-1H-pyrazine 2-Methyl-3-acetylpyrazine |
| Pronunciation | /tuː əˈsiːtɪl θriː ˈmɛθɪl paɪˈreɪziːn/ |
| Identifiers | |
| CAS Number | [5891-22-7] |
| 3D model (JSmol) | `3D model (JSmol)` string for **2-Acetyl-3-Methyl Pyrazine** (C7H8N2O): ``` CC1=NC=CN=C1C(=O)C ``` |
| Beilstein Reference | 135636 |
| ChEBI | CHEBI:134452 |
| ChEMBL | CHEMBL318471 |
| ChemSpider | 120974 |
| DrugBank | DB14145 |
| ECHA InfoCard | ECHA InfoCard: 100.110.666 |
| EC Number | 620-934-3 |
| Gmelin Reference | 152754 |
| KEGG | C10788 |
| MeSH | D000197 |
| PubChem CID | 12306786 |
| RTECS number | UJ8588000 |
| UNII | 5Y2EA03O9B |
| UN number | UN3077 |
| Properties | |
| Chemical formula | C7H8N2O |
| Molar mass | 150.18 g/mol |
| Appearance | Pale yellow to yellow powder |
| Odor | roasted, popcorn, nutty, coffee |
| Density | 1.08 g/cm³ |
| Solubility in water | slightly soluble |
| log P | 1.01 |
| Vapor pressure | 0.000102 mmHg at 25 °C |
| Acidity (pKa) | pKa = 14.51 |
| Basicity (pKb) | 2.40 |
| Magnetic susceptibility (χ) | -51.25·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.533 |
| Viscosity | Liquid |
| Dipole moment | 2.95 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 282.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -105.0 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4256.6 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P272, P273, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P362+P364, P337+P313, P403+P233, P405, P501 |
| Flash point | 60°C |
| Autoignition temperature | 400 °C |
| Lethal dose or concentration | LD50 (oral, rat): > 2000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 >2000 mg/kg |
| NIOSH | HN9843000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.1 mg/m³ |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
2-Acetylpyrazine 2,3,5-Trimethylpyrazine 2,3-Dimethylpyrazine 2-Ethyl-3-methylpyrazine 2-Acetyl-3-ethylpyrazine |