Plenty of people overlook 2,6-dimethylpiperidine when scanning through organic chemistry’s long list of cyclic amines. Back in the mid-20th century, the search for better, more versatile chemical building blocks pushed chemists to pay closer attention to substituted piperidines. N-heterocyclic compounds had worked their way into both industrial and pharmaceutical pipelines, and research into their utility turned up 2,6-dimethylpiperidine’s handy properties. Large-scale production didn't begin overnight; it rode the wave of demand for specialty ligands and tailored intermediates, especially as more synthetic strategies leaned on nitrogen-based scaffolds.
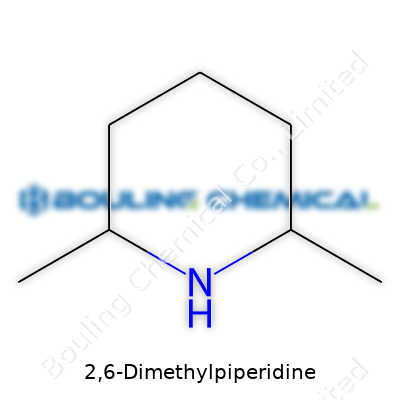
2,6-dimethylpiperidine enters the chemical stage as a six-membered ring sporting two methyl groups on its second and sixth carbon atoms. This structure shapes its behavior and value, mainly by creating steric bulk near the nitrogen atom, altering how it partners in reactions. Both its cis and trans isomers show up in the wild, though labs often prefer the trans-form for certain transformations. Reliable suppliers, usually under demanding quality controls, ship it for chemical synthesis, while specialty outlets cater to researchers looking for a defined isomer.
Pick up a sample and you’ll find a colorless, flammable liquid with a faint, amine-like odor—hard to miss unless your nose spent some time in a chemistry lab. Its boiling point hovers close to 140–150 °C, and it doesn’t readily dissolve in water, yet spreads itself thin in many organic solvents. That ring with two methyl branches bumps up its hydrophobic character, shapes its reactivity, and makes the molecule a little bulkier than simple piperidine. It remains quite stable under standard conditions, though it can take on protons or alkyl groups in the right hands.
Bottles come labeled for purity, isomer ratio, and hazardous nature, with chemical companies using material safety data sheets that flag the fire risk and potential toxicity. Instead of just giving a percentage, reputable sources spell out gas chromatography or NMR readings to give buyers concrete data. For some procedures, trace metal content or water levels make all the difference—a detail not lost on suppliers. The best bottles carry Catalog Numbers and batch numbers, so every drop’s story can be traced from batch to bench.
Chemists often make 2,6-dimethylpiperidine through hydrogenation of 2,6-dimethylpyridine—known as lutidine—with either heterogeneous or homogeneous catalysts. It’s not just a matter of throwing reagents together; catalyst choice, temperature, and pressure not only affect yield, but also the isomer ratio. Some procedures lean on Raney nickel for broad utility, while others drag organometallic catalysts into the fray to fine-tune selectivity. Although the process appears straightforward, reproducibility and purity only come from careful optimization after some trial and error.
Put it in the right flask, and 2,6-dimethylpiperidine refuses to sit quietly. The nitrogen likes to pick up protons, making this compound an effective base in organic transformations. Its methyl groups keep larger reagents at arm’s length, steering selectivity in alkylation or acylation reactions. In transition metal chemistry, it teams up with metals as a ligand, lending both its basicity and steric shield to tune catalysts in a wide range of reactions—take enantioselective hydrogenations or cross-couplings, for example. Its amine can stretch into longer chains, form amides, or swing into reductive aminations, all depending on what’s needed in the flask.
One bottle might carry the name 2,6-dimethylpiperidine; another could read 2,6-lutidine hydride, or simply mention the isomers: cis-2,6-dimethylpiperidine and trans-2,6-dimethylpiperidine. Some catalogs stick to CAS number 504-03-0, so confusion rarely crops up when shopping for the right compound. Tradition in the lab sticks with “dimethylpiperidine” while experimentalists swap stories about the quirks of each isomer.
Anyone working with 2,6-dimethylpiperidine in the lab learns quickly that the scent isn’t the only thing demanding respect. Its moderate toxicity and flammability keep gloves and goggles on standard issue. Adequate ventilation isn’t a luxury—fumes can cause dizziness or irritation, and accidental spills require immediate clean-up, often under the watchful eyes of fume hoods and safety data sheets. Transport regulations treat it as a flammable liquid. Handling protocols line up with what’s used for other low-boiling amines—clear labeling, flame arrestors, and ready access to spill kits. Proper storage—away from oxidizers and direct sunlight—saves a lot of headache down the road.
This little molecule works above its weight class. In pharmaceuticals, chemists build more complex drugs by using 2,6-dimethylpiperidine as a protective group or to block certain reaction sites, owing to its bulky, stubborn side chains. In homogenous catalysis, it often shows up alongside transition metals, fine-tuning how reactions unfold. Agrochemical labs dabble with it as an intermediate for products protecting crops. Materials science has started peeking its way, searching for amines to shape polymers with better mechanical and thermal properties. Academic researchers poke and prod to find new avenues—ligands for asymmetric catalysis, or as bases that resist over-reaction.
New methods for synthesizing 2,6-dimethylpiperidine show up all the time, promising higher selectivity and less waste, especially as green chemistry takes the spotlight. Pairing up with automation, labs have designed routes with lower energy demands and tighter control over by-products. Some researchers push its limits in catalysis, hunting for ways to boost atom efficiency or to open up routes that struggle with more slender amines. The conversation’s rarely about making the compound itself, but rather how acting as a bulky, non-nucleophilic base or uniquely tailored ligand pushes complex transformations from “theoretical” to “practical.”
Toxicology studies on 2,6-dimethylpiperidine, while not as extensive as with everyday solvents, point to moderate acute toxicity and irritant properties. In my experience with related amines, eye and respiratory exposure never fails to cause discomfort. Mouse and rat studies show LD50 values that demand a healthy respect without sparking panic for trace exposure. In cell cultures, the compound interrupts normal metabolic processes at sufficiently high concentrations. Long-term exposure data, especially for industrial handlers, runs a bit thin, underlining the need for gloves, goggles, and regular breaks from the fume hood.
Green chemistry and the push for smarter, less wasteful processes will shape what comes next for 2,6-dimethylpiperidine. Its success in making catalysts more selective already buys it respect in the lab, but it could play a bigger role as research focuses on limiting the environmental impact of industrial chemistry. Cleaner synthesis methods and safer derivatives may bring this molecule into new territories, especially when paired with advances in pharmaceutical design and advanced materials science. With each innovation, chemists will keep looking for building blocks that handle tough conditions and keep side reactions to a minimum. 2,6-dimethylpiperidine won’t stand in the front row of everyday industrial chemicals, but in creating efficiency and precision, it keeps earning a spot in the toolbox of synthetic and applied chemists everywhere.
Talking chemistry with friends who tinker in labs, it’s easy to see why some molecules catch more attention than others. 2,6-Dimethylpiperidine falls into that quirky group you spot in textbooks, somewhere between the basics and the more complex nitrogen-based rings. With the chemical formula C7H17N, this compound shapes itself as a piperidine ring decorated with two methyl groups at specific spots along the ring—those are at carbon positions 2 and 6.
Picturing its makeup, I look back at those hexagonal ring diagrams that populate organic chemistry classes. Piperidine itself stands as a six-membered ring with five carbons and a single nitrogen. By adding methyl (-CH3) groups to the second and sixth carbons on this ring, the resulting shape isn’t just another closed loop, but one with a pair of stubs poking out in just the right places. This direct attachment of methyl groups can change how the molecule fits with others, which comes in handy for synthetic chemists building more complicated frameworks.
Thinking about daily chemistry routines, knowing the exact landscape of the molecule—where every atom sits—shapes how researchers choose solvents and reagents. The methyl groups on 2,6-dimethylpiperidine don’t just crowd the ring for decoration; they push and pull at its geometry. In practice, this means 2,6-dimethylpiperidine can pack less tightly in a crystal, giving it a somewhat lower melting point than its non-methylated relative. Out in the lab, that little difference affects how smoothly it dissolves, reacts, or even sits in a bottle on the shelf.
Organic chemists lean heavily on molecules like piperidine derivatives for building blocks. Adding methyl groups slants the chemical toward new possibilities. For example, the extra bulk from the methyls can make this molecule fit less predictably in enzyme pockets, changing reaction speeds and, often, reaction paths. That’s not just classroom theory; it shows up in real experiments aiming for medicines, catalysts, and advanced materials. I’ve watched this play out as colleagues switch methyl placements to tweak drug candidates, seeking a balance between activity, safety, and side effects.
While 2,6-dimethylpiperidine doesn’t appear on every pharmacy shelf, the compound’s popularity keeps steady in industrial chemistry and research circles. One practical snag—its distinctive odor—reminds chemists to handle it with care. Spilling a few drops early in my laboratory days, I learned fast about the importance of ventilation. Synthesis can produce a mixture of stereoisomers, so careful separation follows. The molecule has two “chiral centers,” meaning two ways to organize the attached groups at each methylated carbon. Chemists need to separate these different isomers for high-purity work, often reaching for chromatography or crystallization—techniques that eat up both time and resources.
For chemists aiming to improve results with 2,6-dimethylpiperidine, I’ve noticed value in rethinking separation steps and refining synthetic paths. Using greener solvents, or seeking catalysts that help push one isomer over the other, can shrink waste and cost. Open collaboration among researchers also uncovers fresh tricks, from alternative purification methods to safer handling routines. Small changes, applied consistently, lift both safety and efficiency in labs of every size.
You won’t see 2,6-dimethylpiperidine at the neighborhood pharmacy or even in most specialist supply shops. Yet, this chemical finds its way into a surprising number of labs and plants. The story behind it isn’t one of overnight fame, but of steady reliability. This compound shows up in chemistry as a source of nitrogen, and the presence of two methyl groups gives it just enough bulk to behave a little differently from regular piperidine.
Chemists hunting for sharper, more selective reactions often turn to fine-tuned molecules. 2,6-dimethylpiperidine plays that role. In catalyst development, those methyl groups offer enough hindrance to steer reactions in new directions. That opens up paths to making pharmaceutical building blocks with the necessary purity and configuration. A few talented researchers have pulled more value out of this compound, using it to help create ligands for transition metal catalysis. That means better drug synthesis, less waste, and often easier purification steps. The bottom line: chemistry gets cleaner and reactions get more controlled because of this small molecule.
In the world of medicine and crop science, tiny changes in molecules make a massive difference. Sometimes, just sticking a different ring or side chain onto a scaffold turns a generic compound into a billion-dollar product. 2,6-dimethylpiperidine gives medicinal chemists a way to tweak structures when searching for new drugs or pesticides. Its six-membered ring balances flexibility and rigidity, and that opens up analogs otherwise tough to create. More than once, a team has reported a lead compound that only came about because someone tried a piperidine variant instead of the classic raw material.
Ask any organic chemist about handy tools, and building blocks like 2,6-dimethylpiperidine always come up. It acts as a base to mop up unwanted acids during reactions. Small shifts caused by the two methyl groups often help steer outcomes, leading to cleaner main products and fewer byproducts. This saves both time and money in labs that count every penny spent on wasted chemicals. I’ve watched grad students frustrated by impurities switch to this nitrogen-base and suddenly see better yields and far less gunk in their final products.
These six-membered rings show up in the design of new polymers and specialty materials. Sometimes, tweaking a side group with 2,6-dimethylpiperidine brings improvements, such as better flexibility or resistance to breakdown. Working in material science means always testing new tweaks, and this molecule gives just the right amount of bulk to change a material’s feel or life span. The paint and coatings sector, for example, sometimes taps unusual amines to fight weathering, and this one makes the shortlist for durability testing.
Demand for custom molecules keeps growing, especially as industries push for greener processes and smarter medicines. As more groups look for ways to cut waste and boost specific outcomes, expect to see more of these modified piperdines in patents and new products. Anyone who’s spent time at the bench knows there’s always another experiment that could use a fresh twist. Chemicals like 2,6-dimethylpiperidine don’t make headlines, but they do push boundaries from the shadows.
2,6-Dimethylpiperidine isn’t some sluggish, rock-solid compound you can stash in the back of a supply closet. It holds a strong amine odor and can turn volatile under the wrong conditions. Most folks working with this chemical know that you don’t want it sitting out where vapors could drift through the workplace. Vapor exposure can irritate the eyes, nose, or throat, and accidents spike when people cut corners. I’ve seen old labs with cracked containers or loose caps, and that’s a recipe for leaks and strong chemical fumes.
Temperature sits front and center in this story. High heat kicks up evaporation risks. 2,6-Dimethylpiperidine prefers a dry, cool spot away from direct sunlight and any source of warmth. If temperatures climb, pressure can build up inside storage bottles. That’s how spills start or, in the worst case, bottles burst their seals. I always make it a point not to keep amines near hot equipment or under sunlight-facing windows.
Tight sealing makes all the difference. I’ve watched folks trust crumbling plastic or ancient glass, but all it takes is one drop of moisture or air sneaking in. The liquid absorbs water from the air, leading to possible contamination and headaches down the line. Store it in high-quality containers with airtight lids, clearly labeled and dated, so you know exactly what you’re grabbing. If the bottle gets sticky or crusty, it's time for disposal, not recycling.
Many labs keep incompatible substances apart, but in busy spaces, bottles sometimes creep together. 2,6-Dimethylpiperidine doesn’t get along with strong oxidizers, acids, or bases. One accidental contact can set off heat or fumes, even in a closed cabinet. Assign a specific place for amines, separate from other reactive chemicals. I make sure storage maps get updated every time supplies move around, especially after big stock orders.
Working with organic chemicals involves risk, but responsible teams don’t play catch-up. Spend time training anyone who might handle the compound, even those who rarely crack open a bottle. Personal protection matters: gloves, goggles, and good ventilation. Fume hoods shut out exposure and draw off vapors before they cause problems. I’d rather see someone ask twice about protective gear than try to tough it out.
Spills turn urgent fast with 2,6-Dimethylpiperidine. Absorbent pads and neutralizing agents should sit within arm’s reach, not buried under boxes. Small leaks disappear quickly with the right absorbents, but mopping up with paper towels or old rags could spread vapors further. Disposal matters just as much: waste cans designed for organic solvents, sealed and clearly labeled, heading to specialized disposal rather than general trash.
I’ve learned that the best labs run well because the basics don’t get overlooked. Check seals and labels every week. Replace aging containers. Keep chemical logs updated, and never let amines wander near incompatible bottles. Get everyone talking about storage and handling, not just the lead chemist. The more eyes on the system, the safer the work. It’s less about fancy technology and more about folks caring enough to watch each other’s backs in the day-to-day shuffle of the lab.
Handling chemicals like 2,6-Dimethylpiperidine brings to mind the days I worked in a lab. Folks often underestimate these colorless liquids, but this one hits hard if you treat it carelessly. The ammonia-like smell is more than a warning; it's a sign to get serious about ventilation. Vapor lingers and can sting your nose, eyes, or throat before you realize what’s happening.
Inhalation has always topped my worry list. Even quick exposure can irritate airways or trigger coughing fits. I remember a colleague, a seasoned tech, who once underestimated the time it took to cap a bottle. Within moments, he felt dizziness and burning in his chest. Quick action—opening windows and getting him outside—helped, but it taught our team a lesson about respecting those fumes. Respiratory hazards aren’t just theoretical. Chronic exposure puts people at risk for headaches, lung irritation, and even chemical bronchitis.
People hear “liquid” and think it’s safe on the skin, but 2,6-Dimethylpiperidine burns. Direct contact causes redness or swelling fast. One day, my glove ripped, and a drop hit my finger—nothing dramatic, just a stinging pain and a red spot that lingered through the next day. Gloves, preferably nitrile, are non-negotiable. I wouldn’t dare handle the stuff in shorts or short sleeves. Full coverage is standard, and for good reason.
Any lab veteran knows: eye injuries are brutal. A splash of 2,6-Dimethylpiperidine can mean lasting damage, sometimes beyond repair. Eyewash stations aren’t just for show. Wearing goggles with side shields blocks the splashes that seem to appear out of nowhere when you least expect it.
Flammability slides off the radar for a lot of folks, but not with this compound. The flash point sits low—52°C (125°F)—so open flames and sparks spell trouble. Years ago, an intern set up a flask near a heat gun. One missed calculation, and we were lucky the only casualty was a scorched bench mat. Fume hoods and spark-proof tools are essential.
This isn’t a chemical for the back of a closet or an open shelf. Tight containers, a cool spot, and steady labeling keep things safe. I stick to lockable storage and leave nothing up to memory. Leaks and spills demand an immediate response. Absorbent pads, then soapy scrubbing, not paper towels. Contaminated material never touches regular trash—sealed waste containers go straight to hazardous disposal.
Out of all the lessons I’ve picked up, respect and preparation stand tallest. Before opening a bottle, I check my gear twice, from gloves and goggles to lab coat and mask. Good ventilation remains a quiet hero. I never skimp on time for cleanup or checks. If someone feels sick, we step away and get to fresh air—no toughing it out.
A few simple habits can draw the line between a regular work day and real trouble. Setting up spill kits, reviewing safety sheets, and talking through protocols before starting all make a difference. Each time I work with 2,6-Dimethylpiperidine, I think of those close calls, and remind myself the label warnings mean business. Safety isn’t overkill; it’s survival.
Strolling through a lab supply catalog looking for 2,6-Dimethylpiperidine, the first thing that jumps out is the purity number. Laboratory work doesn’t forgive sloppy materials. For this compound, purity matters because even tiny contaminants can derail reactions or throw off results.
Most reputable chemical suppliers list their 2,6-Dimethylpiperidine around the 98% mark. Sometimes suppliers offer it with a stated purity ranging from 95% up to 99%. Why not 100%? Organic compounds always carry trace byproducts from manufacture or handling. Achieving that three-nines level means spending more time and resources on purification—and with no guarantee those few extra decimals help most chemists. In real-world research, a 98% purity batch has done the job well, even for sensitive syntheses, as long as the application didn’t call for pharmaceutical or electronic grade.
Purity should match task. Large-scale preparations or less-disciplined reactions might make do with the lower end. Tight tolerance work, like asymmetric synthesis research, often pays extra for those higher numbers—even if just for peace of mind. It’s the old tradeoff: buy the best you can justify, but no purer than you need, since costs climb higher per percent.
Anyone who’s unpacked orders knows bulk chemicals rarely arrive in glamorous packaging. For 2,6-Dimethylpiperidine, the most common choices come as small bottles—usually glass or plastic—ranging from just a handful of grams up to half a kilo. The smallest standard for most research suppliers sits at 5 grams, climbing through 25-gram and 100-gram offerings. Larger labs, or those scaling up to run batches by the liter, might order 500 grams or a full kilo, often in heavy-duty HDPE containers.
From experience, the lid matters as much as the label. This liquid doesn’t get along with moisture, air, or rough treatment. Suppliers usually choose sturdy, airtight closures and sometimes toss in a foil seal, since a leaky bottle can ruin a whole shelf. Lightweight secondary cardboard gets the job done, but trying to get the last milliliter out of a narrow bottle always leads to a few grumbles.
Nothing falls through the cracks faster than running out mid-experiment, so it pays to read the fine print. Some companies add up extra charges for amber glass, specialty safety shipping, or temperature control—things that can push costs surprisingly high for a “simple” base chemical. Over the years, finding a good supplier who packs well, labels concisely, and won’t swap bottle style without notice has saved a lot of stress in the thick of a project.
The world of pure chemicals sounds lofty but stays very much grounded in shelf space, cost realities, and ease of handling. For 2,6-Dimethylpiperidine, most labs find what they need in that 98% bracket, bundled up in bottles rarely bigger than a kilo—though big production outfits might chase drum-scale sizes. Upgrading purity sometimes makes a difference, but knowing what fits the task keeps budgets in check. The real trick: making sure that what arrives matches what the bottle says—and that there’s enough left for the next run, sealed tight and ready for work.
| Names | |
| Preferred IUPAC name | 2,6-Dimethylpiperidine |
| Other names |
2,6-Lutidine hydride 2,6-Dimethylhexahydropyridine |
| Pronunciation | /ˈdaɪˌmɛθəl.paɪˈpɛrɪdiːn/ |
| Identifiers | |
| CAS Number | 504-03-0 |
| Beilstein Reference | 1311037 |
| ChEBI | CHEBI:15304 |
| ChEMBL | CHEMBL15461 |
| ChemSpider | 17001 |
| DrugBank | DB03630 |
| ECHA InfoCard | 100.109.498 |
| EC Number | 202-083-9 |
| Gmelin Reference | 79268 |
| KEGG | C06227 |
| MeSH | D053690 |
| PubChem CID | 10454 |
| RTECS number | EK2975000 |
| UNII | 8SJX337J09 |
| UN number | NA1993 |
| Properties | |
| Chemical formula | C7H17N |
| Molar mass | 113.21 g/mol |
| Appearance | Colorless liquid |
| Odor | ammonia-like |
| Density | 0.827 g/mL at 25 °C |
| Solubility in water | slightly soluble |
| log P | 0.96 |
| Vapor pressure | 0.32 mmHg (25°C) |
| Acidity (pKa) | 11.2 |
| Basicity (pKb) | 3.26 |
| Magnetic susceptibility (χ) | \-68.8·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.426 |
| Viscosity | 0.877 cP (20°C) |
| Dipole moment | 1.29 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 168.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -105.9 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4063.7 kJ·mol⁻¹ |
| Hazards | |
| GHS labelling | GHS02, GHS05, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H226, H302, H314 |
| Precautionary statements | Precautionary statements: P261, P280, P304+P340, P312, P405, P501 |
| NFPA 704 (fire diamond) | 1-3-0 |
| Flash point | 52 °C (125 °F; 325 K) |
| Autoignition temperature | 254 °C |
| Explosive limits | 1.08–6.86% |
| Lethal dose or concentration | LD50 (oral, rat): 370 mg/kg |
| LD50 (median dose) | LD50 (median dose): 455 mg/kg (rat, oral) |
| NIOSH | WI6700000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 ppm |
| Related compounds | |
| Related compounds |
2,6-Dimethylpyridine 2,5-Dimethylpiperidine 2,3-Dimethylpiperidine 2,6-Diisopropylpiperidine Piperidine |