Back several decades, chemistry labs saw tests with morpholine derivatives pick up pace. Industry and university researchers, searching for better stabilizing agents and fine-tuning solvent properties, began homing in on dimethyl substitutions. Folks working on textile processing and pharmaceuticals noticed that dialing the chemical structure of morpholine would shift its behavior and open up fresh applications. By the mid-twentieth century, synthesis paths for alkyl morpholines settled into the chemical literature, giving 2,6-dimethylmorpholine a steady foundation for broader industrial uptake. Looking back, it’s clear: the idea that small tweaks to a molecule can unlock big changes in application keeps driving this branch forward.
Talking with chemical suppliers and reading manufacturer data sheets, I often see 2,6-dimethylmorpholine listed as a specialty intermediate. Its role in syntheses—whether for big polymers or small-molecule drugs—reflects its stable, cyclic ether structure, with two methyl groups nudging reactivity in useful ways. Production volume isn’t huge compared to base morpholine, but there’s enough demand from labs and specialty manufacturers to keep it available globally. For end-users, it comes up as a liquid reagent, commonly delivered in sealed metal drums or glass containers sized for research or pilot plant work.
In the bottle, 2,6-dimethylmorpholine appears clear, with a faint amine odor. The added methyl groups raise its boiling point compared to simple morpholine, giving more control in temperature-sensitive synthesis. Its solubility swings with the solvent, favoring polar environments thanks to the ether-amine backbone. Stability under standard storage wins praise, but one lesson sticks: always check compatibility when pairing with strong acids, since nitrogen-containing rings can react and form unwanted byproducts. The density and refractive index of this compound don’t stray far from cousin morpholines, providing a familiar profile for analytical tracking.
Buying chemicals for regulated work means scrutinizing the label for purity. 2,6-dimethylmorpholine typically ships with assay levels above 98%. Labs must verify batch info, supplier lot numbers, and shelf life, since degraded product can introduce inconsistencies. MSDS sheets underline storage ranges—usually below 30°C, away from light—and remind users about its flammability and reactivity. Being mindful of hazard symbol updates and region-specific regulations (like GHS in Europe and the U.S.) saves a lot of back-and-forth on compliance forms.
In graduate school, the route to 2,6-dimethylmorpholine came up in organic synthesis coursework. Nucleophilic amination of 2,6-dimethyl-1,4-dichlorobutane, followed by ring closure, yields the target morpholine. Many small-scale syntheses lean on this sequence, although commercial outfits often tweak steps for better yield or greener reagents. Pressure reactors help compress reaction times and keep air out, since oxidation can foul the product. Precise temperature monitoring and controlled addition of precursors ensure repeatability—a must for large-batch operators.
In real-world chemistry, the versatile ring of 2,6-dimethylmorpholine offers up multiple interaction points. Its nitrogen lone pair behaves predictably, accepting protons or acting as a nucleophile. You see it playing a part in substitution reactions, especially when constructing more complex heterocyclic frameworks. The dimethyl groups act as sentinels, shifting electronic properties and steering regioselectivity. Modifying the ring further for tailored reactivity remains a popular research focus, particularly in pharmaceutical lead design and new catalyst development.
Checking catalogs, 2,6-dimethylmorpholine sometimes goes by alternate names like N,N-dimethylmorpholine (incorrectly, but not uncommon in trade circles) or 2,6-DMM. Chemists use its CAS number to avoid confusion—offering a clear, direct reference that sidesteps local naming quirks. Common international vendors list the chemical under English, German, Japanese, and Chinese equivalents, making procurement more straightforward for cross-border teams.
Long days in the lab teach a person to respect anything with even mild amine reactivity. 2,6-dimethylmorpholine demands gloves and tight-sealing goggles during handling. Vapors can irritate the respiratory tract, so hoods or well-ventilated set-ups become standard practice. If spilled, the liquid slicks across benchtops and floors with surprising spread, underlining the importance of careful bottling and immediate cleanup. Documentation calls out incompatibility with strong oxidizers, and many factories keep automated leak detection for tanks storing this and kindred chemicals. Disposal follows hazardous waste streams, with incineration under controlled air conditions the recommended route.
Conversations with chemical buyers and production managers show 2,6-dimethylmorpholine mostly leaving the factory for specialty applications. Polyurethane catalysts, where subtle changes in catalyst design shift foam properties, benefit from its inclusion. Pharmaceutical research, always hungry for new ring systems, explores it as a building block for anti-infectives or nervous system modulators. It’s found a niche as a solvent for select separations and extraction systems—making use of its polar, but non-volatile nature. Labs synthesizing dyes or corrosion inhibitors list it as a key intermediate for fine-tuned molecular design.
Talking to colleagues in academia, the morpholine core keeps showing up in medicinal chemistry patents and journal articles. Teams look for bioactivity in the dimethyl derivatives, blending computational modeling with bench chemistry. New methods of catalyzing morpholine ring modification crop up across the literature, as green chemistry pushes for milder, less wasteful syntheses. Some interest comes from process engineers trying to shift classic routes into continuous flow production—seeking cost savings and improved safety without sacrificing purity levels. Funded collaboration between universities and specialty chemical manufacturers remains a hotbed for testing fresh function out of old ring structures.
Assessing toxicity means more than just checking the LD50. Animal tests and cellular assays paint a picture of risk for 2,6-dimethylmorpholine, falling somewhere between basic solvents and reactive monomers. Acute exposure brings skin and eye irritation, while long-term effects get less attention—but no smoke means no fire in industrial settings, provided safety measures hold. Toxicologists underline the importance of limiting airborne concentration and controlling batch spills during large-scale use. Regulators watch for breakdown products in wastewater, requiring factories to monitor effluent and install scrubber technology where needed. Long-term human studies remain thin, so best practice treats this as a strictly industrial compound, with little exposure justified outside controlled environments.
Looking ahead, the scientific community keeps poking the morpholine structure for new tricks. Advanced polymer science, especially for low-emission insulation or medical-grade foams, may call for tailored catalysts that 2,6-dimethylmorpholine can help deliver. Environmental chemistry has begun to study substitution patterns to lower ecological impact, hinting at greener degradation profiles compared to past morpholine derivatives. Drug discovery looks set to circle back to this ring system for ideas as resistance profiles shift and older therapies lose ground. As industries look for ever-tighter process control and specialty function, chemistry rooted in direct lab experience with 2,6-dimethylmorpholine shapes both its current use and emerging directions.
Once you start working with chemicals and industrial processes, you run into all sorts of specialty compounds. 2,6-Dimethylmorpholine is one of those lesser-known chemicals that plays a surprisingly important role in the background of manufacturing and synthesis. People rarely hear about it outside of chemistry circles, but its reach is much broader than most of us imagine.
Most times, 2,6-Dimethylmorpholine turns up as a catalyst in making polyurethane foams. Polyurethane has become nearly invisible to most consumers because it pops up everywhere—furniture, car seats, insulation, and plenty of everyday products. In the foam-making process, you want chemistry that nudges the reaction forward without leaving unwanted leftovers. This compound steps in to help speed up the transformation of raw materials into flexible foam, making production smoother and cutting down on waste.
With more demand for energy-efficient homes and vehicles, manufacturers look for ways to get better performance from insulation and cushioning. If you've ever noticed how some foam feels softer, lasts longer, or holds up under heavy use, you can thank the chemical recipe behind it. Companies keep tweaking their blend of ingredients, often using catalysts like 2,6-Dimethylmorpholine to fine-tune the results. It helps make products more consistent in quality, which means fewer failures and returns.
Beyond foams, it also plays a role in organic synthesis. Chemists reach for 2,6-Dimethylmorpholine when they're working with complex molecules, especially in pharmaceuticals and specialty coatings. It can help rearrange molecules, tie pieces together, or break down bigger compounds. This function makes it valuable in drug development or when companies want to test new coatings or adhesives that outlast the competition.
Whenever a lab needs a reliable building block for experiments or production, this compound often sits on the shelf. I’ve worked in a materials science lab where minor changes in catalysts made products sturdier or simpler to produce. Many times, a swap like this helped keep projects on schedule and saved money by reducing the number of update cycles.
Chemical safety keeps coming up for a good reason. Compounds like 2,6-Dimethylmorpholine demand respect. Though not considered high-risk for consumers at the product’s end-use stage, handling in large batches can create risks. Workers need training and personal protective equipment to avoid issues. I’ve seen what happens when someone overlooks safety steps—a simple mistake can lead to chemical burns or fumes that affect air quality.
Incidents like these make better safety protocols more important than ever. Regulators and manufacturers invest in training and modern containment systems so workers don’t pay the price. Good record-keeping and regular inspections also prevent most major accidents. By putting safety first, companies protect workers and keep operations running reliably.
As environmental standards get stricter, companies search for ways to reduce chemical waste and emissions. Sustainability teams have been evaluating everything from raw materials to the energy used in factories. There’s growing interest in recovering or recycling catalysts, and 2,6-Dimethylmorpholine fits into this conversation. Sourcing from responsible suppliers and adopting closed systems can both trim the ecological footprint of manufacturing.
Overall, the unassuming role of this compound underlines a big trend—innovation depends on both the science and the responsibility behind it. Chemicals like 2,6-Dimethylmorpholine will keep playing their part as manufacturers rethink how products are made, used, and recycled.
2,6-Dimethylmorpholine, with its formula C6H13NO, brings together an interesting set of elements: carbon, hydrogen, nitrogen, and oxygen. People might overlook compounds like this, but every molecule carries its own set of possibilities. Morpholines have a longstanding place in both industrial and laboratory chemistry, and tweaks to their structure, such as the addition of methyl groups at the 2 and 6 positions, can totally change how they interact with other chemicals and materials.
Through a little bit of work in industrial labs, I've seen chemicals like 2,6-dimethylmorpholine turn up in places you wouldn’t expect. It's not just about mixing stuff together; it's about how even a small shift in molecular formula directs the road map for what you can build. Those methyl groups affect both the basicity and the solubility, which in turn influence the kind of reactions it can participate in. You find these morpholine derivatives in the development of pharmaceuticals, rubber accelerators, and corrosion inhibitors. The smallest tweaks open up entirely new uses.
Nobody who has spent time handling these compounds ignores how a single molecule can impact human health or the ecosystem. Any time I've worked with nitrogen-containing organics, ventilation and personal protective equipment become priorities. It isn’t just good practice, it's about responsibility. The structure of 2,6-dimethylmorpholine means that it can be both reactive and persistent, so its journey isn't finished when it leaves the lab bench. Companies run up against stricter regulatory boundaries all the time, especially once persistent organic pollutants hit the radar.
Some people see chemical nomenclature as nothing but a memory test, but that misses the point. A simple formula like C6H13NO actually puts a lot of knowledge in your hands. It's short for six carbon atoms arranged to support not just any ring, but a morpholine ring, methylated at two positions, with that characteristic nitrogen-oxygen backbone that can accept or donate electrons. That kind of understanding drives innovation—once the structure is clear, it’s easier to design safer derivatives or more effective industrial processes. Teaching newcomers how to break apart or visualize chemical formulas sets them up for confident work in real-world settings.
Safety education must stay hands-on. No policy or guideline replaces experience—simulated spills, measuring vapor concentrations, reviewing reactivity tables. I’ve watched seasoned chemists avoid mistakes because they asked simple questions about what their chemicals could really do according to formula. Filtering knowledge through experience turns the periodic table from a list into a tool kit. Manufacturers should keep their safety data plain, up-to-date, and accessible. Pushing for green chemistry methods and recycling approaches also matters, reducing both waste and hazard. Every minor substitution in the molecular build, such as adding two methyl groups, invites everyone to question, test, and go further in their chemical problem-solving.
Taking the time to understand basics like the formula of 2,6-dimethylmorpholine opens new ways to solve the old problems. Whether in safer manufacturing, investigating uses in synthesis, or reducing environmental footprints, a familiarity with these building blocks helps both seasoned professionals and new learners push for improvement. Science leans forward on curiosity and a willingness to make small changes—sometimes, the right answer comes from looking closely at the details that others skip over.
Familiarity with industrial chemicals can turn into a double-edged sword. Spend any time working in manufacturing or a research lab, and you’ll spot bottles labeled 2,6-Dimethylmorpholine sitting near solvents and coatings. To most folks outside of these industries, the name sounds obscure, maybe even a little intimidating. Yet, chemicals like this underpin paints, plastics, and pharmaceuticals—a fact that tends to slip past public conversations.
Manufacturers rely on 2,6-Dimethylmorpholine as a building block, especially for making polymers and resins. Its usefulness stems from its molecular backbone, helping form sturdy materials that don’t fall apart under heat. Workplaces recognize that materials with complex-sounding names often demand a healthy skepticism. It’s not about fear-mongering; it’s about respecting the fact that many industrial chemicals carry risks that aren't immediately obvious to the naked eye.
Step into any chemical storage room, and you’ll run across safety data sheets stacked with hazard statements. Inhalation of 2,6-Dimethylmorpholine vapors can cause significant irritation of the nose and lungs. Even brief skin contact poses problems, leading to rashes or burns. Folks exposed daily in factory settings learn quickly not to skimp on gloves, goggles, or even fume hoods.
The Environmental Protection Agency and the European Chemicals Agency flag substances like 2,6-Dimethylmorpholine for a reason. Acute exposure can knock a worker off their feet with headaches or dizziness, while repeated misuse increases the chance of liver or kidney damage. One study out of Germany tested morpholines on lab rats and noted evidence for organ toxicity, though direct links to humans remain scarce. Gaps in research may lull some into downplaying the risks, but that attitude only raises the stakes.
Training and equipment make a real difference. My time shadowing a veteran plant operator taught me that confident handling grows out of repetition and education. He never skipped a training session, no matter how long he’d worked with industrial amines. His stories of colleagues shaken by splashes or spills stuck in my mind; just because something’s routine doesn’t mean it’s harmless. A splash that lands on bare skin leads to days of discomfort, not just a bad afternoon.
Proper lab ventilation, clear labeling, and updated safety protocols help cut down on accidents. Regular review of safety data keeps risks from fading into the background. Workers have a right to know what chemicals they touch. Watching a team pause before opening a new drum, double-checking labels and pulling up the latest safety sheets, makes it clear: respect for chemistry keeps people healthy. Personal protection isn’t a formality—it's what sends everyone home in one piece.
Government agencies have their hands full regulating chemical exposures. Improving transparency between employers and employees should take priority, especially by making data about long-term health risks easy to find. Manufacturers can invest in research on safer alternatives. Labs that experiment with substitute compounds tend to find new ways to reduce both environmental impact and workplace injury. Safety isn’t about halting progress but about picking paths that recognize human health as non-negotiable.
Chemicals like 2,6-Dimethylmorpholine stay necessary for modern industry. That reality means we owe workers—and the environment—a commitment to stay vigilant, to learn, and to invest in the smartest protections available.
Few people enjoy discussing chemical storage, yet any experienced lab tech or plant manager knows trouble starts with small mistakes. I remember my early days in a busy materials lab. A mislabeled jug led to a faint smell in the air and a room full of groans as we scrambled to identify the source. That lesson stuck—chemicals deserve respect and routine, especially the organic types that react easily to heat, moisture, or oxygen.
This compound, widely used as a solvent and an intermediate in chemical synthesis, can be hazardous without the right setup. Its vapor irritates the eyes and lungs and, if heated, can catch fire. Locking these risks out of day-to-day work keeps people safer and prevents costly downtime.
Not every shelf in the warehouse works for every bottle. These morpholines call for a cool, dry spot that rarely crosses 25°C. Moisture can cause slow reactions and break down the chemical long before its expiration date. A steel cabinet with good ventilation, grounded for static control, fits the job. Avoid putting it near oxidizers, acids, or sources of heat. I once watched a fire marshal shake his head at a box of chemicals left close to an old electric heater. The fines cost much more than better shelves ever would.
Glass offers chemical resistance and keeps air and water out. Polyethylene works for smaller volumes if it’s high-grade, but cheap plastics break down. Top up each bottle so there isn’t much air left above the liquid. Oxygen sneaking in through the cap starts a slow but steady breakdown even if the bottle stays closed.
You can’t stop an accident if half the building has keys to the chemical store. Keep access strict. Sign-in and sign-out logs add accountability. I’ve seen storage rooms where one rotating logbook alone stopped midnight chemistry projects that skirted safety rules. If nobody knows who took what, cleanup after an incident gets complicated.
Spills are not rare, even in careful labs. Ready-to-go kits with absorbent pads, gloves, and neutralizers cut risks in half. Place eye wash stations and showers within easy reach, too. Every time a new hire joins, walk them through a drill—more than once, I ran into new team members who skipped the shelf of safety gear because “no one ever showed me.”
Set a reminder each quarter to check labels and check seals. Date each container after opening. Don’t let old material pile up in the back of your storage. Many accidents I've read about started because expired chemicals stayed hidden until a shelf collapse or routine cleaning day.
Every bottle of 2,6-dimethylmorpholine depends on a few good habits and a little vigilance. Reliable storage cuts risks, saves money, and keeps everyone on the right side of safety and the law. No one ever regretted spending too much time organizing the chemical store, but plenty have regretted cutting corners.
2,6-Dimethylmorpholine isn’t a compound you hear about every day outside of chemical circles, yet its physical properties shape how researchers, manufacturers, and handlers interact with it. This colorless liquid looks harmless at first glance. Pour it into a beaker and its clear, almost watery consistency is hard to distinguish from many ordinary solvents found in a lab. But dig deeper and the story changes.
If you’ve ever worked with organic solvents, boiling point is one of the first properties to check. 2,6-Dimethylmorpholine boils at about 170°C. In a world full of volatile chemicals, this is on the higher end for small amine derivatives. The higher boiling point means it hangs around in liquid form during many synthetic processes, which reduces loss through evaporation. That matters if you’re thinking about cost or environmental control. Not everything with a nitrogen or oxygen in its ring behaves as courteously under heat.
Vapor pressure connects closely to these thermal properties. It stays relatively low under typical laboratory conditions. Anyone working in a hot shop, near open vessels, or without robust ventilation should still respect its ability to slowly build in enclosed airspace. Experience has taught that even lower-volatility compounds can surprise you over a long shift or in summertime labs.
2,6-Dimethylmorpholine mixes well with water; solubility runs high due to the hydrophilic nature of the morpholine ring. Don’t let the methyl groups fool you—they get pushed aside compared to the oxygen and amine functionalities. This property means the compound disperses rapidly in aqueous environments. If spills happen, cleanup becomes less difficult, assuming the correct safety procedures are followed. I remember a case where a beaker tipped over and, thanks to its mixing ability, the residue became manageable with routine dilution and absorption.
Not all solvents announce their presence with sharp scents. 2,6-Dimethylmorpholine’s odor stays faint and a little amine-like, but it doesn’t sting the nose like some simpler amines. Personal protective equipment—gloves and goggles—still matter, since skin and eye irritation risks don’t disappear. Handling this compound without proper gear once gave me a tingle that lasted the afternoon. These little lessons turn into habits: double-check the gloves, don’t lean too close to the flask, and keep that face shield handy.
While not as flammable as many smaller organics, 2,6-Dimethylmorpholine still burns under the right circumstances. Its flash point hovers near 62°C. In practical terms, this rules out open flames and calls for grounded storage containers. No one wants to see what a morpholine fire looks like—they produce thick, unpleasant smoke and demand a dry chemical extinguisher for a safe response.
Developing policies around chemicals like 2,6-Dimethylmorpholine starts with appreciating its everyday physical behavior. With a melting point close to -55°C, it doesn’t solidify under regular conditions, which means leaks and exposure can go unnoticed without active monitoring. Real professionalism in a lab or plant takes attention to these subtle details. Relying on published data, reading the label, swapping stories with colleagues: these practices shape real-world outcomes.
Solid safety starts by knowing boiling points, watching vapor buildup, understanding water solubility, and respecting even the mildest warning odors. No property exists in isolation, and seasoned professionals combine the physical facts with lived experience to keep things running smoothly every shift, every day.
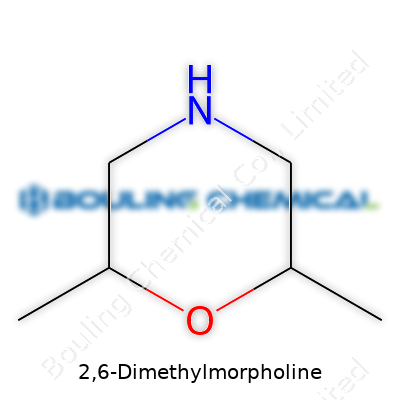
| Names | |
| Preferred IUPAC name | 4,6-Dimethylmorpholinе |
| Other names |
2,6-Dimethyl-4-morpholine 2,6-Dimethyl-1,4-oxazinane 2,6-Dimethylmorpholine Morpholine, 2,6-dimethyl- |
| Pronunciation | /ˌtuː.sɪksˌdaɪˈmɛθ.əl.mɔːˈfɔː.liːn/ |
| Identifiers | |
| CAS Number | 6365-59-1 |
| Beilstein Reference | 1720529 |
| ChEBI | CHEBI:85256 |
| ChEMBL | CHEMBL49280 |
| ChemSpider | 14179 |
| DrugBank | DB08649 |
| ECHA InfoCard | 13-2119180212-52-0000 |
| EC Number | 2160-24-7 |
| Gmelin Reference | 9274 |
| KEGG | C06167 |
| MeSH | D03823 |
| PubChem CID | 12249 |
| RTECS number | BU1400000 |
| UNII | G8T8Y4GPG7 |
| UN number | UN2264 |
| Properties | |
| Chemical formula | C6H13NO |
| Molar mass | 115.18 g/mol |
| Appearance | Colorless liquid |
| Odor | Amine-like |
| Density | 0.89 g/mL at 25 °C (lit.) |
| Solubility in water | miscible |
| log P | 0.2 |
| Vapor pressure | 0.9 mmHg (20 °C) |
| Acidity (pKa) | pKa = 8.36 |
| Basicity (pKb) | 4.99 |
| Magnetic susceptibility (χ) | -62.2×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.428 |
| Viscosity | 0.88 cP (20°C) |
| Dipole moment | 2.74 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 322.2 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -285.4 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -4233.4 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS05, GHS07 |
| Signal word | Warning |
| Hazard statements | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. Harmful if inhaled. May cause respiratory irritation. |
| Precautionary statements | P280, P303+P361+P353, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 2,2,0 |
| Flash point | 57 °C (135 °F) |
| Autoignition temperature | 190 °C |
| Explosive limits | 1.3–9.8% |
| Lethal dose or concentration | LD50 (oral, rat): 2790 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat 2590 mg/kg |
| NIOSH | SKC03240 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 ppm |
| IDLH (Immediate danger) | IDLH: 1,000 ppm |
| Related compounds | |
| Related compounds |
2,3-Dimethylmorpholine 2,5-Dimethylmorpholine 2,6-Dimethylpiperidine 2,6-Lutidine Morpholine |