Chemists first paid attention to 2,5-Dimethyl Pyrazine in the early decades of the 20th century. It didn’t pull the spotlight right away. Scientific curiosity, mixed with the blossoming field of organic flavor compounds, grew through the 1960s and 1970s. Labs chasing the essence of roasted coffee, chocolate, and toasted grains stumbled across the unmistakable aroma of pyrazines. By working with simple ingredients under carefully controlled heat, they unveiled a family of compounds central to natural and artificial flavors. Work on methylated pyrazines flowed from food science into other corners of chemical research. Academic labs, especially in Japan, Germany, and the United States, slowly built comprehensive reports on its synthesis and sensory qualities. This laid a foundation for both industry-scale flavor creation and a deeper understanding of the chemical principles behind flavor chemistry, setting the tone for product innovation through the late 20th century and beyond.
2,5-Dimethyl Pyrazine doesn’t spend time in glossy advertising campaigns, but industry professionals recognize it as a staple scent and flavor additive. It’s colorless or pale yellow, favoring those who want to enhance baked, nutty, or roasted notes without throwing off other flavor balances. Producers usually sell it in liquid form, measured out with precision in aromatic compositions. Ubiquity often translates to reliability: 2,5-Dimethyl Pyrazine rarely hides unwanted quirks, making it a predictable ingredient in both small-batch craft processes and large-scale manufacturing. In daily practice, food engineers reach for it any time a product needs that extra punch of toastiness, depth, or “freshly baked” authenticity, whether in snacks, sweet goods, or even pet foods.
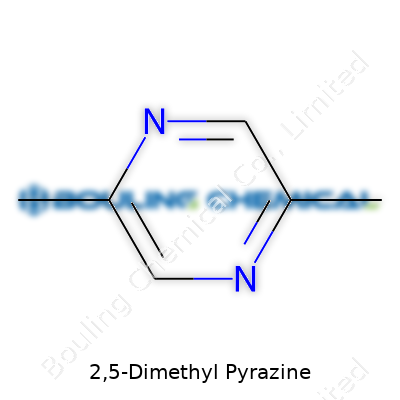
This compound offers a melting point around 56–58°C and boils near 168°C, giving it a solid presence in both heated and room-temperature processes. Structurally, it shows the signature six-membered pyrazine ring substituted with methyl groups at the 2 and 5 positions. The addition of methyl groups stabilizes its aroma’s roasted, nutty edge. It resists hydrolysis under neutral conditions and dissolves readily in alcohols and organic solvents but only sparsely in water. With a relatively low molecular weight, volatilization under moderate heat is guaranteed, making it perfect for applications where controlled aroma release matters. Its refractive index hovers around 1.492, and the density averages close to 1.06 g/cm³, which means measurement and mixing present no great hassle for manufacturers.
Commercial lots of 2,5-Dimethyl Pyrazine generally guarantee a purity above 99%. Analysts look for near absence of lead, arsenic, and solvents, aiming for food-grade standards. Labels on drums and smaller packages spell out batch numbers, production dates, and best-by data points. Transport documents follow regulations from the United Nations and local authorities, keeping worker safety and public health in view. MSDS sheets warn of potential irritant qualities, as expected with concentrated aroma chemicals. Many vendors comply with FEMA GRAS status and receive Kosher and Halal certification when markets demand more than just flavor impact. Lab technicians run checks for low moisture content and a refractive index within a certifiable range, reducing the risk of spoilage or unexpected reactions.
Early researchers started by reacting ammonia with diacetyl or methylglyoxal under pressure, producing a crude yield of various methylated pyrazines. Today’s manufacturing setups use more refined, reproducible routes. Many producers prefer cyclization of butanedione and ammonia with subsequent methylation, carried out under controlled, anhydrous conditions. Sodium or potassium hydroxide often steps in as a basic catalyst, and the process might run under inert atmosphere to prevent side-product formation. Yields matter more for economics than for craftsmanship in industry, so engineers optimize for reaction temperature, time, and feedstock purity. Separation by distillation and extraction removes minor impurities, sharpening both aroma and stability. Industrial processing takes care to ventilate and capture volatile organic byproducts, keeping production lines safe, clean, and compliant with environmental laws.
2,5-Dimethyl Pyrazine’s aromatic ring invites substitution and functionalization, opening doors for chemists wanting to tinker with sensory properties. Halogenation, Friedel-Crafts alkylation, and selective oxidation can introduce more complexity or “fine-tune” a profile for a narrow application. Adding larger or polar substitutes changes volatility and flavor nuances, guiding the hand of a flavorist or scent chemist searching for new variants. In most standard settings, the structure prefers stability, but with the right catalyst, oxygen, or strong acid, it transforms or breaks down into related molecules. The trick lies in orchestrating reaction speed and selectivity so that only the desired positions shift, and the original roasted charm sticks around or evolves in planned ways.
Chemists flip between 2,5-Dimethyl Pyrazine and names like 2,5-Dimethyl-1,4-diazine or simply “DMP.” Industry catalogs often bundle it under FEMA 3273 or CAS #123-32-0. Specialty suppliers craft branded variations for premium sensory lines or high-purity requirements, but most in the trade just ask for “dimethylpyrazine” or use short codes and abbreviations. In some Asian and European countries, localized names may appear in regulatory or technical literature, but cross-trading always circles back to these basics when clarity or import paperwork counts most.
Manufacturing and handling protocols prioritize both worker health and product control. The aromatics may irritate eyes, skin, or respiratory tract in concentrated form or if mishandled. Facilities enforce fume hoods, gloves, and eye protection in the processing and packing areas. Storage guidelines call for cool, dry spaces, away from strong oxidizing agents. Fire risk stays minimal except under rare, high-heat scenarios. Shipping containers often display the UN number for traceability and hazard communication. Regular audits and in-house monitoring help keep exposure within safety limits, referencing standards set out in OSHA, REACH, or national equivalents. Spills and cleanup rely on standard absorbent materials and disposal procedures, seldom raising complications when teams stick to established checklists.
Food industry experts turn to 2,5-Dimethyl Pyrazine in flavoring cereal, bakery, chocolate, and roasted nut products where distinct, full-bodied “brown” notes elevate consumer experience. Some companies use it in instant coffee, snack coatings, or processed cheese. The pet food sector leans on it to make kibble more appealing, working closely with animal nutritionists to balance palatability and safety. Beyond food, perfumers dabble with small amounts to create warmth or “edible” complexity in fragrances. A few researchers probe into its use for masking unwanted off-notes in supplements or pharmaceuticals, though regulatory rules bring added scrutiny for these applications. Wherever food scientists want a golden-bake signature—shortbread, malt, or nut—the compound shows its worth.
Current R&D moves in two directions: refining synthesis for energy efficiency and pushing the boundaries of aroma blending. Academics probe the diverse range of pyrazine analogs, testing sensory responses using advanced analytical instruments and trained tasting panels. Industrial labs hunt for biosynthetic routes, including microbial fermentation, which could sidestep petrochemical dependencies and meet demand for “natural” labeling under evolving consumer regulations. Chemometric approaches help sort through structural variants, with machine learning models mapping connections between molecular tweak and sensory output. Brands committed to clean-label movements invest in residue analysis, aiming to keep the ingredient list familiar and trustworthy. Collaboration across chemistry, sensory science, and environmental engineering drives smarter, safer, and greener pathways to the same bold flavors consumers crave.
Toxicologists have mapped the boundaries of safe exposure for over forty years. Standard animal studies point to low acute toxicity by oral, dermal, or inhalation routes at levels way above those found in finished foods. In cell lines, evidence suggests only minimal irritation or mutagenic potential—always at far higher concentrations than anyone encounters in real-world use. Regulators including the FDA and EFSA recognize it as safe in food when used as intended. Still, safety reviews pop up every few years to keep up with new research or shifts in industrial usage. Allergic reactions appear rare, though some workers in concentrated handling environments report mild symptoms after repeated exposure, suggesting a case for continued monitoring and best-practice protections on the factory floor.
Food trends focus on bold, authentic, and “minimalist” labels, and 2,5-Dimethyl Pyrazine stands ready to support this movement. Consumer demand for sustainable ingredients nudges manufacturers to invest in bio-based synthesis, drawing from renewable feedstocks and greener chemistry. Digital flavor modeling and simulated mouthfeel studies open doors for custom blends that fit ever-tighter taste profiles, pushing the ingredient further into the specialized, boutique segment. Expect to see more collaborations across food tech startups, fermentation specialists, and flavor houses, all vying to make classic roasted notes more natural and traceable. Patents keep stacking up, covering everything from new synthesis protocols to tailored ester derivatives, ensuring the storyline on this compound has many more chapters. Advances in lightweight process sensors, combined with sharper quality analytics, push both safety and creative application higher for next-generation foods and consumer products.
Walk into almost any food factory, and odds are, someone’s got a container of 2,5-Dimethyl Pyrazine tucked away on a shelf. This is the compound that brings a roasted, nutty punch to plenty of snacks. You’ll find it behind the irresistible scent of toasted nuts, crackers, cocoa, and even some coffee blends. I remember spending afternoons at a friend’s bakery, wondering how fresh bread could taste even nuttier than usual. Turns out, flavors like these often come from this tricky little pyrazine boosting the aroma.
It doesn’t mimic hazelnuts or peanut butter outright, but it gives food companies a reliable way to start with bland flour and end up with treats that seem to have spent all day in the oven. Given how many consumers expect bigger flavors without more sugar or fat, pyrazine bridges the gap by packing more taste without upping the calories.
Head over to the tobacco industry and you’ll see another side to this molecule. Cigarette and cigar makers sprinkle it into blends to mask harshness and coax out mellow, robust notes. It’s no secret that people want less bitterness in their smoke, so flavor chemists reach for pyrazine to tune out the acrid edge. For those working up new booms in vaping and e-cigarette juices, the molecule shapes profiles that echo roasted coffee or caramel, bringing those familiar comforts even outside the food world.
Stepping away from food, perfumers use a tiny drop of this compound to round out earthy, woody scents. You won’t sniff a bottle and think “roasted nuts,” but it softens sharper notes, nudging the final blend toward comfort and familiarity. Believe it or not, I once worked with a candle maker who explained how a dash of pyrazine created a “warm room” vibe. Customers responded to that subtle backdrop, even though nobody could quite name where the coziness came from.
Its structure lets it linger and survive processing heat, so it holds up well during baking or roasting. The molecule nestles into recipes and formulas, stays stable, and keeps doing its job from the start of production all the way to the finished product in your hand. Labs have noted its low odor thresholds—meaning it takes barely any to affect the senses—so it’s cost effective to boot.
With so much use, safety questions pop up, especially as people care more about what’s in their food and air. The FDA currently considers it safe for foods in small amounts, but there’s always pushback from those who want every ingredient strictly natural. New research sometimes brings up health worries for inhaled flavors in vaping products, so responsible manufacturers check usage rates and source materials carefully.
If companies are looking for cleaner labels, natural sources, or flavor alternatives, they could put more money into finding pyrazines that come straight from nuts or roasted veggies, instead of making them in a lab. Better communication with consumers—explaining what each ingredient does and why—can build trust, instead of leading to confusion.
On the surface, it just sounds like another chemical, but 2,5-Dimethyl Pyrazine has its fingerprints all over modern flavor and fragrance. That craving for roasted coffee or the comfort of peanut brittle owes more to this compound than most people realize. Anyone curious about what gives packaged nuts that boost or makes a scented candle feel like home would do well to pay attention to the world of pyrazines—and to keep asking questions about what’s behind the taste and scent of everyday favorites.
Walk down any supermarket aisle and the smell of roasted nuts, fresh bread, or a chocolate bar grabs your attention instantly. 2,5-Dimethyl pyrazine sits behind many of those toasted notes that make snacks crave-worthy. Food companies like this compound because it can lift the aroma and taste of many snacks, sauces, and seasonings. For those of us who enjoy roasted coffee or nutty granola, we've probably tasted a trace of it without even knowing.
Regulators in big markets such as the US and Europe have put this ingredient through plenty of filter tests. The US Food and Drug Administration (FDA) recognizes it as “generally recognized as safe” (GRAS) for food. The European Food Safety Authority (EFSA) takes a similar stance, letting the substance appear in flavorings so long as the amounts reflect natural conditions and don't exceed certain limits. These approvals didn't happen quickly, and researchers looked at how much people eat, what happens after it's digested, and whether it builds up over time. The safety data covers animal studies, consumption patterns, and chemical breakdowns in the body. So, on paper, food products containing this flavor molecule line up with rules and safety standards right now.
It always pays to think twice before writing off public concern about food additives, even after regulatory clearance. Many folks hear “artificial” and get uneasy. Some people have allergies, sensitivities, or worry about long-term health because the food industry keeps adding new compounds to processed snacks. In my own eating habits, I've picked up the habit of reading ingredient lists out of simple curiosity, and sometimes out of concern for my young family. While I might trust the science, I also see how everyday people want fewer unknowns in what they eat.
Research so far doesn’t point to serious risks at the amounts found in food. Most studies using much higher doses in animals did not show any scary effects. 2,5-Dimethyl pyrazine tends to break down fast after eating, and the body pushes it out. About the only clear risk crops up if someone tried to consume big amounts at once, which typical packaged foods never provide. Still, every so often, food scientists turn up questions that spark new studies, especially when compounds show up in more and more products.
Trust gets built on open, clear information. Every time new additives hit the shelves, companies and regulators should lay out research in plain language. For 2,5-dimethyl pyrazine, companies that talk openly about sourcing and testing give consumers more peace of mind. Ongoing research helps, too—the more we check these flavoring agents as food habits shift, the better our understanding. If food makers pulled back on heavy use and kept flavor blends simple, people would feel even safer.
People care a lot about what goes into their bodies and their children’s lunchboxes. Taking a closer look at ingredient lists, asking for better labels, and choosing snacks with fewer complicated names helps put more power into consumers’ hands. As someone who sits at the dinner table with a growing family, I know the importance of being able to relax about the food we share. No one wants to play guessing games about what’s in their meals. With clear information, steady monitoring, and honest communication between food makers and families, trust in food can be stronger.
Big chemical suppliers list 2,5-Dimethyl Pyrazine with purity levels that hover around 98% to 99%. It's rare to spot anything lower than 97%, especially in catalogs aimed at food, fragrance, or research industries. Above this mark, costs increase sharply, but for most uses, companies stop at 98-99%.
There’s always a drive for higher purity, especially in fields where trace impurities can throw off both the smell and the safety profile of the compound. A batch going to a food flavoring business, for example, usually shows a certificate with the purity, and often a breakdown by gas chromatography. This transparency builds trust with buyers, especially those in regulated sectors.
I've seen how a lab-grade or food-grade tag on the bottle doesn’t always mean absolute perfection. 2,5-Dimethyl Pyrazine carries a nutty, roasted aroma, and even a tiny contaminant can twist the scent or flavor. I once worked alongside a flavorist who could taste a difference using a so-called “pure” sample that registered slightly below spec. The tweaking of recipes to mask off-notes can cost time and money, so a reputable supplier matters as much as the stated purity.
Pharmaceutical or analytical work turns the spotlight on purity even more harshly. Lab techs lean on high-purity stock to chase down faint signals in testing, or to be sure a single unlisted contaminant won’t skew the results. Breaching the 99% mark gets expensive, but in these environments, it's often non-negotiable.
Impurities don’t just muddy the sensory profile; some have real risks. Certain byproducts formed during synthesis—notably in older factories or when cutting corners—may be harder to remove. Even if a supplier claims 98%, a good lab still checks for specific possible contaminants if the use is sensitive. My background in process engineering taught me that problems often start in the details others overlook: equipment cleaning, solvent grade, or storage. A careless move at any step leaves traces behind. This makes paperwork and independent lab checks a critical part of business, not just bureaucratic hurdles.
Not every sector needs maximum purity. Bulk applications, like in animal feed, have different standards than those for fine fragrances or medicines. Still, if a company gets complaints or labs spot off flavors, they have to track the issue right down the supply chain. This is where documentation and spot-checking matter, not just for one shipment but batch after batch. Audits, both planned and surprise, push companies to stay honest about their process. Some buyers will even request a small sample before buying large amounts, letting them do in-house testing for assurance.
On the technical side, advances in purification methods, like column chromatography and molecular distillation, help clean up the product even further. Better process control—automated temperature and reaction monitoring—keeps fewer byproducts from forming. Investment here isn’t just about legal requirements. Customer trust and ongoing business hang in the balance.
Anyone sourcing 2,5-Dimethyl Pyrazine with confidence looks at the supplier’s history, the paperwork, and does their own spot checks. It’s about predictability. Whether blending flavors, developing a new consumer product, or running complicated lab tests, knowing every bottle will act the same builds the strong relationships companies want with both their suppliers and customers.
2,5-Dimethyl pyrazine has a familiar toasted, nutty scent that pops up in coffee, roasted nuts, and even popcorn. Food technologists and flavor houses reach for it to add warmth and complexity to their products. But anyone who’s handled this compound knows it doesn’t like surprises. Room temperature can tip the balance from crisp aroma to a dull, lifeless whiff.
I’ve seen it: open a bottle exposed to sunlight for a few weeks, and what was once sharp turns flat. The science behind it goes back to the same rules that protect your fresh bread or your favorite spice blend. Light, oxygen, and heat break things down. They spark reactions, sometimes silent and slow, that cut into quality. The nose always notices.
The best trick is to keep the bottle away from light. Amber glass containers shield the liquid much like sunglasses for the beach. While working in a small-scale lab, I always reached for the darkest bottle. Take the hint: pyrazines do best in the dark.
Oxygen brings trouble too. Once that seal breaks, the clock starts ticking. Using a tightly closed cap after every use isn’t just nitpicking; it stops air from worming its way inside and altering the chemistry. Some stockists in the industry go one step further—using nitrogen-flushed vials. You might not need this at home or in every workplace, but it’s not excessive in bulk storage.
Heat shortens shelf life. I learned this lesson the hard way, storing a handful of flavor samples above a radiator. Summer heat or winter mistakes push the compound closer to breakdown. Keep it cool, not freezing, usually under 25°C. Refrigeration isn’t a bad idea for long-term storage, as long as moisture doesn’t sneak its way in. Condensation creates its own mess, and water mingles poorly with pyrazines.
One detail often missed is the label. Dates matter. Marking the opening date helps track freshness, and regular checks go a long way. I’ve tossed more than one sample that picked up an off-note over time. Routine audits—sniff tests and checks for cloudy appearance—keep products safe and pleasant.
For smaller operations, I’ve noticed a tendency to buy too much “just in case.” That strategy backfires. Smaller quantities ordered more often usually give better results, less waste, and fewer apologies when product launches run up against a musty batch.
More investment in proper containers and clear routines saves money and headaches. It’s not about expensive technology, but about consistency and care. Sharing storage space with pungent chemicals or constant exposure can spoil a whole drum. Segregating flavor chemicals by reactivity and strength keeps the unique character of each one intact.
Training staff, new hires, or interns avoids common failures. Small reminders—such as always using lined lids and resealing immediately—keep the routine smooth and quality high. Anyone with a nose for flavor knows it only takes one slip to ruin weeks of careful work.
Holding onto the best possible aroma in 2,5-dimethyl pyrazine isn’t about luck. It’s common sense applied with discipline, built on regular habits and respect for the tools of the trade.Every so often, I stumble across a request for a simple string of digits—a CAS number. These numerical identifiers seem pretty dry on the surface, but dig a little deeper and you find how much they shape safety, quality, and progress in labs and factories worldwide. Ask a chemist about 2,5-Dimethyl Pyrazine and they’ll likely rattle off its CAS number: 123-32-0. It’s a set of numbers that cuts through language barriers, confusion, and even risky mistakes.
I don’t often think about obscure chemicals in daily life, yet aroma lovers and food scientists swear by 2,5-Dimethyl Pyrazine. This molecule shapes the smell and flavor of roasted nuts, coffee, bread crusts—the scents floating through bakeries and kitchens. These intense cravings and emotional responses stem from a compound with a simple, stable identifier that links global product chains. The CAS number guarantees certainty; one missed digit could mean the difference between the warm comfort of roasted coffee aroma and a completely different chemical with unknown effects.
Plenty of businesses don’t just need an ingredient—they need to be sure they’ve got the right one. Food labs, perfumers, and even regulatory offices depend on clear identification. I've seen it happen where a simple confusion in a paperwork trail leads to a stockroom full of the wrong material. Companies don't want to risk tainted products or regulatory fines, so they rely on those three sets of numbers. For 2,5-Dimethyl Pyrazine, typing 123-32-0 into a supplier database pulls up the same compound every time, across borders and brands. Safety data sheets, formularies, and transport labels all line up with that number.
Working in any lab, there’s always a risk that bottles get swapped or records aren’t clear enough. It doesn’t take more than a moment’s distraction for things to go sideways. I remember a colleague misreading a tiny print label, only to realize just in time the chemical inside wasn’t what was listed in the notebook. Standardizing with CAS numbers helps, but only if everyone throughout the system pays close attention and follows the rules.
People are the crucial link. Most issues start with people, too. Training goes a long way. Some organizations run regular courses that show how to spot errors and double-check CAS numbers at every touchpoint. Another approach is tech: good inventory systems scan barcodes linked to CAS data, helping to catch errors before they cause trouble. Keeping chemical records up to date and using digital checklists tightens the system further.
It's easy to breeze past a series of digits like 123-32-0, yet these numbers anchor real life in research, production, and trade. I keep reminding myself how much hassle gets saved with these small steps. The everyday world of chemicals, food flavors, and safety owes a lot to a shared language as simple as a verified CAS number. For 2,5-Dimethyl Pyrazine, those digits open doors and keep doors shut—protecting people, processes, and products with a minimum of fuss.
| Names | |
| Preferred IUPAC name | 2,5-Dimethylpyrazine |
| Other names |
2,5-Dimethylpyrazine 2,5-DMP Pyrazine, 2,5-dimethyl- UNII-2C99K019WO |
| Pronunciation | /tuː,faɪv-daɪˈmɛθ.ɪl paɪˈreɪziːn/ |
| Identifiers | |
| CAS Number | 123-32-0 |
| Beilstein Reference | 717934 |
| ChEBI | CHEBI:34783 |
| ChEMBL | CHEMBL330243 |
| ChemSpider | 6990 |
| DrugBank | DB04230 |
| ECHA InfoCard | 100.094.591 |
| EC Number | 121-84-8 |
| Gmelin Reference | 82297 |
| KEGG | C14368 |
| MeSH | D016721 |
| PubChem CID | 13788 |
| RTECS number | XU5950000 |
| UNII | Y3G1Y8Y9FJ |
| UN number | UN2386 |
| CompTox Dashboard (EPA) | DTXSID0024462 |
| Properties | |
| Chemical formula | C6H8N2 |
| Molar mass | Molar mass: 108.14 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | nutty; roasted; cocoa |
| Density | 1.034 g/mL at 25 °C (lit.) |
| Solubility in water | Slightly soluble |
| log P | 0.7 |
| Vapor pressure | 0.38 mmHg (at 25 °C) |
| Acidity (pKa) | pKa = 3.79 |
| Basicity (pKb) | 1.60 |
| Magnetic susceptibility (χ) | -43.3·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.4970 |
| Viscosity | 1.029 mPa·s (at 25 °C) |
| Dipole moment | 1.68 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 253.4 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -2.73 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3251 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | Precautionary statements: P261, P305+P351+P338, P312 |
| Flash point | 56 °C |
| Autoignition temperature | 430 °C |
| Explosive limits | Explosive limits: 1.4–11% |
| Lethal dose or concentration | LD50 (oral, rat): 940 mg/kg |
| LD50 (median dose) | LD50 (median dose): 460 mg/kg (oral, rat) |
| NIOSH | PY2560000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.5 mg/m³ |
| Related compounds | |
| Related compounds |
2-Methylpyrazine 2,3-Dimethylpyrazine 2,6-Dimethylpyrazine 2,3,5-Trimethylpyrazine 3,5-Dimethylpyrazine |