Chemists have long looked to pyrrole derivatives as a playground for unlocking reactivity and pushing the boundaries of organic synthesis. 2,5-Dimethyl-1H-pyrrole took shape over a century ago, following a wave of pyrrole research that spanned the late 1800s into early modern organic chemistry. Patent filings from the dye and pharmaceutical industries showed this compound was never just an afterthought—labs studied its core structure for reactivity, color properties, and as a jump-off point for designing more complex molecules, especially as researchers chased hemoglobin mimics and synthetic dyes. Academic and industrial teams in Europe and North America took turns refining its synthesis and mapping out its behaviors. Many chemists who championed simple heterocyclic design in the early days would probably recognize their influence in today’s libraries full of methylated pyrroles. Over generations, its utility grew with advances in chemical instrumentation, which allowed for better purity, identification, and attention to safety, marking it as a mainstay for both research and industrial interest.
2,5-Dimethyl-1H-pyrrole shows up in labs and plants where people want a small, robust, and versatile nitrogen-containing ring. Its basic template—a five-membered pyrrole with methyl groups at the 2 and 5 positions—earns it a reputation as a starting material for more ambitious targets. Companies sell this compound in varied forms, typically boasting a purity north of 97%, and researchers see it as a favorite building block in the search for new drugs, advanced polymers, and specialty dyes. Suppliers have followed demand by offering it in volumes that serve both experimentation and small-batch manufacturing, making it available and accessible for both routine use and groundbreaking projects.
The structure gives the molecule both character and challenge. With the molecular formula C6H9N, 2,5-Dimethyl-1H-pyrrole lands as a colorless to pale yellow liquid, though exposure to air and light can lead to darkening. Its characteristic odor betrays its pyrrole roots—earthy, sometimes reminiscent of roasted coffee beans, a feature that shows up because of the nitrogen in the ring. This compound boils at approximately 141-143°C, with a melting point well below room temperature, so it stays liquid unless chilled to near freezing. Its moderate solubility in common organic solvents like ether, benzene, and alcohols allows for easy chemical manipulations, but water tends to reject it, so it floats atop any attempts at aqueous blends. The dual methyl substituents boost its lipophilicity and slightly reduce its reactivity compared to unsubstituted pyrrole, nudging it towards specific uses. The compound is sensitive to light and air, leading to polymerization on standing, which means users must store it tightly closed, under an inert atmosphere, and protected from light.
Industry standards for 2,5-Dimethyl-1H-pyrrole demand tight control over contamination and precise labeling practices. Certificate of Analysis sheets list not just purity, but moisture content, residue after ignition, color, refractive index, and storage requirements. The labeling—usually featuring CAS number 625-84-3—puts a spotlight on flammability, self-polymerization risk, and reactivity with oxidizing agents. Suppliers rate it for laboratory use or industrial application, and warn against inhalation hazards and direct skin or eye contact. Container labeling often advises refrigeration or protection from air and light, so users can keep batches stable between projects.
Several established methods exist for making 2,5-Dimethyl-1H-pyrrole, but most scale-up operations favor the Paal-Knorr synthesis. This method relies on the cyclization of hexane-2,5-dione with ammonia or an ammonium salt under acidic conditions, producing the desired ring with excellent efficiency. Some routes use reductive cyclization from diketones, taking advantage of cheap, available feedstocks, while others explore organometallic routes for more selective outcomes. Post-synthesis, the crude mixture faces careful distillation to remove side products and stabilize the product, because even minor impurities can trip up further reactions or taint analytical data. Purification by vacuum distillation or careful chromatography helps keep quality high. Each approach has trade-offs in terms of raw material cost, reaction complexity, and ease of scalability, so the choice tends to rest on project goals and regulatory demands.
2,5-Dimethyl-1H-pyrrole gives chemists an easy entry point for many reactions—amidation, halogenation, and even straightforward oxidation are all accessible. The methyl groups at the 2 and 5 positions block direct substitution on those carbons, steering incoming groups to the remaining positions on the ring and offering a measure of selectivity. Classic Vilsmeier-Haack formylation introduces formyl groups at position 3, while the nitrogen atom accepts alkyl chains or protects groups without too much trouble. Polycondensation leads to extended conjugation, which drives modern organic electronic applications. The electron-rich ring resists harsh conditions, but extended exposure to oxygen will quickly darken the material, as polymerization sets in. Synthetic chemists tap the pyrrole’s reactivity to produce advanced intermediates, cross-coupled compounds, and even conductive polymers, which broadens the compound’s reach far beyond fundamental organic chemistry.
Manufacturers and catalogues use a handful of accepted names to refer to this molecule: 2,5-dimethylpyrrole and 2,5-lutidine-pyrrole come up often, while chemical registries fixate on its IUPAC name, 2,5-dimethyl-1H-pyrrole. Less standardized terms show up in translated literature or supplier lists, but consistent CAS identifiers cut through confusion. Regulatory filings and safety data sheets stick to these core names, ensuring buyers and handlers always talk about the right compound, even across different geographies or supplier systems.
Handling this pyrrole takes thought and respect for chemical safety. Vapors can irritate eyes, nose, and lungs, and liquid spills pick up speed when ambient temperatures climb. Gloves, goggles, and fume hoods stand as standard protocol for chemists at the bench or pilot-plant operators. The compound catches fire easily, so storing it away from heat and open flames is essential. Containers need a tight seal and a cushion of nitrogen or argon to shut out oxygen. Clean-up needs an absorbent material and should avoid water, as the compound floats and spreads easily, raising the perimeter of any spill quickly. Waste solutions go through solvent recovery or incineration to avoid environmental escapes, following tough disposal regulations for nitrogen-containing organics. Chemists learn to respect the darkening or thickening of their pyrrole bottles, knowing those are signs of slow polymerization and contamination that can trigger runaway reactions or compromise future experiments.
2,5-dimethyl-1H-pyrrole stakes out territory in fine chemical synthesis, dye development, advanced polymers, and attempts to mimic natural porphyrins—the core scaffold of hemoglobin and chlorophyll. Functional chemists turn to it when designing molecules for organic light-emitting diodes and solar cell components, because its structure resists rapid degradation while still enabling electron flow in conjugated systems. Dye chemists value the tailored reactivity for building new pigments with improved stability or novel color properties, especially for applications where customization matters more than mass-market affordability. In recent years, pharmaceutical teams have re-examined it as a template for small-molecule enzyme inhibitors. Its core structure pops up in patents exploring insecticides, fungicides, and specialty anti-corrosion treatments. Every industry that cares about robust nitrogen heterocycles keeps a close eye on how to wring new value out of this old workhorse.
Academic labs and R&D departments gravitate toward 2,5-dimethyl-1H-pyrrole for its versatility in synthetic strategies and as a model compound for studying aromaticity and electron delocalization. Grant-funded projects use it to probe reaction mechanisms, while industry research teams riff on its structure to break new ground in optoelectronic materials, particularly for thin-film electronics and organic semiconductors. Some teams blend it into organic frameworks for sensing devices or push for more eco-friendly pigment production. Instrumental advances in NMR, mass spectrometry, and crystallography fuel new discoveries, as researchers identify subtle reactivity patterns and clue into ways to harness the methyl groups for further chemical elaboration. Teams looking for greener manufacturing turn to catalysis, improving atom economy and reducing waste, while still producing high-purity product suitable for downstream applications. The worldwide pipeline continues to push new patents and literature reports, exploring every niche where the basic pyrrole skeleton can be pushed or pulled to deliver value.
Toxicity sits at the intersection of chemistry and public health. 2,5-dimethyl-1H-pyrrole doesn't loom large on lists of notorious toxics, but responsible use demands a full accounting. Animal studies point to moderate acute toxicity with respiratory and mucous membrane irritation, though chronic effects have not been exhaustively documented. Workers in facilities using the compound often undergo routine air monitoring and spirometry to avert sneaky health impacts, and safety data sheets always stress swift clean-up of spills and prompt ventilation of any area where vapors build up. In the broader environment, it's not as persistent as other industrial organics, yet run-off could still trip up aquatic systems if careless disposal occurs. Regulatory guidelines push for low exposure and proper protective equipment. As the scientific world moves toward more sustainable chemistry, detailed studies on its environmental footprint and routes of degradation will only increase. Smart money looks to regular toxicological updates as manufacturing and use scale up, keeping worker and environmental protection as central goals.
If past performance predicts future results, 2,5-dimethyl-1H-pyrrole can expect a long, productive career in both established and emerging industries. Its place in active pharmaceutical ingredient synthesis, materials science, and specialty organic chemistry remains secure, but new frontiers open up as demand for advanced electronics and green chemistry rises. Research into functionalized pyrroles for organic solar cells, OLEDs, and next-generation sensors continues to ramp up, with 2,5-dimethyl derivatives often taking center stage because of their reliability and ease of handling. Each year brings fresh patents exploring tweaks to boost performance or introduce new features. Green synthesis routes will grow in importance, prompting investment in catalytic pathways and bio-based feedstocks. As new safety standards and environmental regulations tighten across global supply chains, product stewardship will demand even better data on reactivity, toxicity, and life cycle impact. Those with experience in handling and innovating with this compound see opportunity in adapting time-honored chemistry to solve tomorrow’s technological and regulatory challenges, ensuring 2,5-dimethyl-1H-pyrrole continues to earn its place on every serious chemist's shelf.
2,5-Dimethyl-1H-pyrrole doesn’t make headlines like some bigger-name chemicals, but its reach runs wide in research and manufacturing. During my years in chemical research, small molecules like this one consistently show up where real innovation happens. The compound—easy to spot thanks to its simple structure—brings a unique mix of versatility and reactivity. That power translates straight into its main uses.
Color chemistry might look flashy, but it relies on building blocks like 2,5-dimethyl-1H-pyrrole. This molecule helps create porphyrins and substituted pyrroles—the backbone of many advanced dyes. Lab experience taught me that stringing together simple units often leads to molecules that selectively absorb and emit light. In the last decade, chemists have turned to this pyrrole to advance solar cell dyes and imaging agents. The structure nudges color stability and brightness, making it valuable in both research and industrial labs.
Data back this up. Porphyrin-based dyes pulled from 2,5-dimethyl-1H-pyrrole form the main “antenna” materials in organic solar cells. These cells grab more sunlight and feed clean energy projects. Many of today’s commercial dye-sensitized solar panels use molecules from this chemical family.
Drug discovery hunts for structures that show both creativity and reliability. While working in medicinal chemistry, I saw pyrrole units packed into candidate drug molecules, especially those chasing cancer or bacterial targets. 2,5-Dimethyl-1H-pyrrole lifts up the core scaffold, tweaking molecular flexibility and electronic properties. Its small size lets researchers add it onto a parent structure without making molecules too bulky for the body to absorb.
Market research points to a steady rise in new antitumor and antimicrobial agents built around this scaffold. Adding methyl groups at the 2- and 5- positions blocks unwanted reactions, shaping safer and more effective compounds. Journal studies in recent years highlight how these modified pyrroles outperform older analogs in lab tests—pointing toward a future with fewer drug side effects.
Progress in electronics keeps shrinking circuits, and that leans on custom molecules like 2,5-dimethyl-1H-pyrrole. This chemical forms a springboard for organic semiconductors, which turn up in flexible displays, wearables, and smart sensors. With a touch of heat or an electric jolt, pyrrole-based compounds move charge efficiently. That matters for both device speed and long-term reliability.
Colleagues in industrial R&D often mention that little changes to the pyrrole ring lead to big shifts in conductivity and stability. So, researchers test new blends each year, steering chemical tweaks through 2,5-dimethyl-1H-pyrrole to make faster and more robust sensors. These sensors run in everything from glucose monitors to pollution alarms.
The value of 2,5-dimethyl-1H-pyrrole doesn’t rest on its abundance, but on how smartly it’s put to use. Chemical safety stands at the core: I’ve seen labs grow much stricter with how such pyrroles get handled, especially as demand grows in healthcare and electronics. For anyone in these spaces, making strong partnerships with suppliers and investing in up-to-date safety training proves crucial.
Technology never stands still, and neither do the ways this molecule serves real-world problems. Universities, manufacturers, and labs eager to clean up the electronics and medicine supply chains turn to 2,5-dimethyl-1H-pyrrole as a workhorse and a launchpad for advanced research.
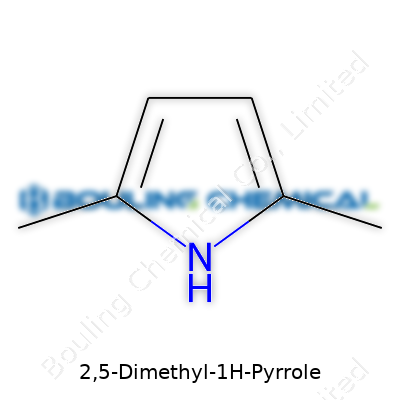
Chemistry often carries a puzzle in every name. Looking at 2,5-Dimethyl-1H-Pyrrole, a few core facts stand out. The “pyrrole” backbone anchors the molecule as a five-membered ring with one nitrogen atom. The “dimethyl” portion marks out two methyl groups joining that ring, each attached at the 2 and 5 spots. Here, every piece of the name hints at the shape, bonds, and chemistry you’re likely to face in practical situations.
In the lab, 2,5-dimethyl-1H-pyrrole grabs attention with its simple, sturdy ring. The five members count four carbons and a single nitrogen, drawing together with alternating single and double bonds. Each methyl addition at positions 2 and 5 stacks a -CH3 group onto the ring, changing both its chemistry and physical properties. Sketching it out, the structure comes alive: two sides stretched with methyls, nitrogen tucked into the ring, and electrons shifting through the framework.
Count up all the atoms and the formula lands at C6H9N. That’s six carbon atoms, nine hydrogens, and just one nitrogen. Some may find it useful to know which carbon gets what. The methyls latch onto carbons next to the nitrogen and directly opposite it, helping stabilize the ring. Visualizing the connections builds a foundation for understanding how this molecule reacts—and why it’s part of broader families in both natural and synthetic chemistry.
Small structural tweaks, such as adding methyl groups onto a pyrrole ring, set off a cascade of changes in behavior. These changes show up in reactivity, how the molecule handles charges, and how it blends into larger, more complex compounds. In research and manufacturing, 2,5-dimethyl-1H-pyrrole routinely serves as a starting block for more advanced materials. For example, researchers exploring conductive polymers or specialized dyes reach for pyrrole derivatives—including this one—since they offer new electronic properties without requiring exotic sources.
Chemists often worry about stability when dealing with small nitrogen heterocycles. Substituting methyl groups helps anchor the molecule, cutting down on easy oxidation. This might not sound dramatic to the non-specialist, but in manufacturing and storage, greater stability means less spoilage and fewer surprises. My own experience working with pyrrole rings reinforces this: a methyl here or there, and suddenly the compound stops breaking down in the bottle before you’re ready to use it.
Looking for better performance or new functions could push future research to test other groups or positions on these rings. Adding electron-donating or withdrawing groups sometimes unlocks unexpected behaviors. Collaborative research benefits from wide access to well-characterized compounds like 2,5-dimethyl-1H-pyrrole, driving innovation in everything from organic electronics to pharmaceuticals.
Clear understanding of both structure and formula forms the foundation of safe lab work, effective synthesis, and development of new materials. As science and technology move forward, solid knowledge like this turns from trivia into an essential tool—helping researchers worldwide push boundaries without the confusion that comes from uncertain structures or properties.
Working in a chemistry lab for years, you realize how quickly sloppy storage habits can lead to sticky situations. 2,5-Dimethyl-1H-pyrrole brings its own set of quirks. Keep it away from air, moisture, and strong sunlight. This compound tends to oxidize fast when left in the open, which can ruin entire batches of material. I’ve seen people lose valuable product because they disregarded simple advice: seal it tightly and keep it cool.
Don’t store it anywhere near heat sources or outside recommended temperature ranges. Chemical manufacturers often suggest refrigeration or at least temperatures below 25°C (room temperature), and from my experience, this minimizes decomposition. Laboratories that follow these guidelines tend to see fewer leaks, strange odors, and unexpected reactions. High humidity and unstable temperatures often speed up unwanted changes in pyrrole-based compounds. Good dry storage pays off.
2,5-Dimethyl-1H-pyrrole gives off a distinctive odor, and inhalation isn’t healthy over long periods. Make a habit of working under a fume hood, even if only for transferring small amounts. Nitrile or neoprene gloves offer decent protection against skin contact, and lab coats should not just hang on the back of a chair. After some mishaps, our lab introduced spill cleanup kits specifically for heterocyclics like this one—quick response makes all the difference when drops go astray.
In daily lab work, contamination sneaks up. Someone once managed to spill pyrrole near a water bath, and the mess reacted with the moisture, turning sticky and brown. Desiccators and low-humidity environments just make life easier. Every container must carry a clear label, and double-bagging glass vials inside a secondary sealed plastic bag helps contain fumes. This may sound strict, but routine makes memory. My mentor always said: “Handle bottles the same way every time, and accidents nearly disappear.” From what I’ve seen, that holds up.
If you have experience in any research or industrial lab, fire safety drills start early. 2,5-Dimethyl-1H-pyrrole catches fire at relatively low temperatures and can form flammable mixtures with air. It’s tougher to put out these fires than to prevent them. Avoid open flames, hot surfaces, and oxidizers. Once, a colleague left a flask near a Bunsen burner; the ensuing rush to smother a small fire taught everyone in the room to check their workspace more often. Good ventilation and working in explosion-proof areas keeps these risks low.
Disposing of pyrrole and contaminated waste properly matters just as much as careful storage. Never pour unused product down the drain. Specialized chemical waste containers and scheduled pick-ups maintain both environmental and personal safety. Having these routines not only keeps the workspace running but protects communities outside the lab as well.
A strong safety culture develops from consistency, not big rules handed down once. Share experiences of close calls or observed leaks, so newer team members learn the hard-won lessons from those before them. Update standard operating procedures as new insights emerge. Emphasize safety inspections, reward staff who spot poor practices before they cause trouble, and always review incident reports real-time—not weeks later. This steady, methodical approach helps keep 2,5-Dimethyl-1H-pyrrole from causing harm and gives researchers peace of mind.
My time in academic research brought me into contact with many chemical reagents, from the relatively tame to the downright dangerous. 2,5-Dimethyl-1H-pyrrole does not stand out in terms of public awareness, but that can add to its risks. Amateur chemists and professionals alike sometimes overlook hazards when a chemical seems obscure. Having worked with several pyrroles, familiarity should never translate to carelessness.
This chemical comes with a set of clear health risks. Like many pyrrole derivatives, it can irritate skin and eyes—and inhaling dust or vapor exposes the respiratory system to possible damage. The molecule’s volatility means accidental spills or open containers quickly create vapor. Burning sensations or rashes can develop rapidly. Once, someone in my lab got a splash on their glove, thinking nitrile would suffice, only to find it seep through after a few moments. Always change gloves at the first sign of breakthrough.
Scientific sources and chemical safety data sheets flag fire hazards, pinpointing a low flash point and rapid flammability. Flammable substances require tight control over ignition sources. Years ago, a neighboring lab stored a bottle near a light fixture; a short circuit sparked a minor fire. Even with no major harm, scorched workbenches and thick smoke drove home that chemicals sometimes punish complacency.
Short-term symptoms grab immediate attention, but repeated or chronic exposure should stay on the radar. Hand-to-mouth contact and poor lab hygiene open doorways for low-level poisoning. Pyrroles sometimes affect the liver and central nervous system, so minimizing exposure helps keep unknown risks at bay. Reading scholarly articles, I’ve learned to check more than MSDS sheets—digging through journal reports reveals effects beyond what's mainstream.
Standard lab procedures work for a reason. Goggles, gloves, and fume hoods offer real protection—strict routine goes a long way. Scratches and hangnails give chemicals entry points. Sometimes, overlooked corners of a workspace host spills, so keeping benches clean attends to both safety and efficiency.
Good ventilation trumps makeshift solutions. My old lab’s window fan proved less than ideal. Mechanical fume hoods with consistent airflow reduce the risk of breathing in low levels of vapor. Never decant volatile substances near open flames or hot surfaces. Even unplugged equipment can harbor enough static to set off a small fire.
Old containers with yellowed caps or poor labeling invite danger. I have seen confusion between similar-looking bottles because someone skipped a step in the labeling process. Locking up flammable chemicals and providing up-to-date, clear identifiers drops the risk drastically.
Unused product and contaminated waste require proper disposal, not a trip to the nearest sink or regular trash bin. Local regulations about disposal exist for a reason—waste handling mistakes can lead to pollution or even chemical burns for unsuspecting custodians.
People working with 2,5-dimethyl-1H-pyrrole don’t need to panic, but they do need to respect what’s in front of them. Thorough training, solid routines, and clear storage plans tackle most hazards. Staying informed—whether by reading up-to-date research, watching for safety guideline changes, or talking with experienced colleagues—keeps small mistakes from becoming big problems.
Respect creates safety. Awareness—grounded in lived experience—remains the sharpest tool in a chemist’s kit.
In the world of research and chemical manufacturing, purity shapes outcomes. Scientists working in pharmaceuticals, organic synthesis, or materials science rely on a consistent and uncontaminated compound to avoid setbacks. For 2,5-Dimethyl-1H-pyrrole, high purity often means 97% or greater. Producers list this as “purity by GC” or “purity by HPLC.” Purity influences both the reliability of new chemical pathways and the trustworthiness of analytical data. Over the years, colleagues and I have learned that cutting corners on purity can mean costly experiment failures or worse—dangerous side products that muddy results.
Buyers shopping for 2,5-Dimethyl-1H-pyrrole face choices that depend on workflow, not just price. Researchers usually need small packages—1 gram, 5 grams, maybe 25 grams—to test hypotheses and assemble complex molecules. Even small differences in packaging can mean fewer headaches in the lab, since over-quantities often spoil over time or need extra disposal efforts.
Large scale users—fine chemicals producers, developers of advanced electronics, research institutes—tend to order in bulk. Suppliers regularly offer 100 grams, 500 grams, or even multi-kilogram packs. Larger drums (1 kg or more) turn up in regions where pilot programs or custom manufacturing grow. Many companies seal packs in amber glass bottles or tightly capped high-density plastics to shield contents from light and moisture.
From a practical standpoint, the way this pyrrole ships matters. Sensitive compounds, especially aromatic heterocycles, degrade with air and water. My own experience has shown that freshly opened material keeps its burgundy color best and produces more consistent reactivity than old, improperly stored chemicals. More than once, I’ve come across labs that stored 2,5-Dimethyl-1H-pyrrole in clear bottles and ended up with degraded or useless material. Proper labeling and safe storage—dark, airtight containers, cool shelves—go beyond box-ticking. They protect both the scientist and the science.
Purity certificates support traceability. Reputable suppliers include a certificate of analysis, showing exact percentages, sometimes even trace impurity data. Researchers can cross-check this to avoid ambiguity and replicate results between orders. Such rigor shields investments and builds trust. Overlooking documentation has led to wasted hours and repeated experiments for teams I advise.
Questions about available grades and sizes often come up because not every supplier lists options clearly. Improving supplier transparency—online catalogs, clear cut sizes, batch traceability—helps researchers plan ahead. Few things stall innovation like running short or receiving a size too big for the refrigerator.
Some in the community are looking at pooling resources using consortia or research clusters. Group purchases let smaller labs get better prices and fresher product, cutting down on waste and expense. Co-ops and procurement groups have started to catch on, especially where universities and start-ups share core facilities.
Labs—large and small—benefit from strong supplier relationships and up-to-date safety data. Quick access to the right size, high purity, and credible certificate brings trust and smoother experiments. The ideal chemical source is one that matches actual volume needs, keeps quality up, and listens to feedback when issues arise. Those basic, human exchanges—an email, a phone call, a clear label—often make the biggest difference.
| Names | |
| Preferred IUPAC name | 2,5-dimethyl-1H-pyrrole |
| Other names |
2,5-Dimethylpyrrole 2,5-Dimethyl-1-pyrrole NSC 55327 |
| Pronunciation | /tuː,faɪv-daɪˈmɛθɪl-wʌn-eɪtʃ-paɪˈroʊl/ |
| Identifiers | |
| CAS Number | 625-84-3 |
| 3D model (JSmol) | 2DWDdCIEuBrpDBPA |
| Beilstein Reference | 120924 |
| ChEBI | CHEBI:33869 |
| ChEMBL | CHEMBL3171047 |
| ChemSpider | 73953 |
| DrugBank | DB04397 |
| ECHA InfoCard | 100.019.465 |
| EC Number | 203-659-4 |
| Gmelin Reference | 77772 |
| KEGG | C06318 |
| MeSH | D016581 |
| PubChem CID | 13614 |
| RTECS number | UJ8575000 |
| UNII | 543UW1A6H7 |
| UN number | UN2810 |
| Properties | |
| Chemical formula | C6H9N |
| Molar mass | 97.15 g/mol |
| Appearance | Colorless to yellow liquid |
| Odor | amine-like |
| Density | 0.911 g/mL |
| Solubility in water | insoluble |
| log P | 1.7 |
| Vapor pressure | 0.49 mmHg (at 25 °C) |
| Acidity (pKa) | 17.0 |
| Basicity (pKb) | 6.44 |
| Magnetic susceptibility (χ) | -42.83·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.5050 |
| Viscosity | 0.883 cP (25°C) |
| Dipole moment | 1.52 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 246.6 J/mol·K |
| Std enthalpy of formation (ΔfH⦵298) | 99.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2953 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H226, H315, H319, H335 |
| Precautionary statements | P210, P261, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 2,3,0 |
| Flash point | 53 °C |
| Autoignition temperature | 385 °C |
| Lethal dose or concentration | LD50 (oral, rat): 3150 mg/kg |
| NIOSH | WS5600000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.02 ppm |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Pyrrole 1-Methylpyrrole 2-Methylpyrrole 3-Methylpyrrole 2,4-Dimethylpyrrole 2,3,5-Trimethylpyrrole |