Chemistry has produced a fair share of odd-smelling compounds over the years, and 2.5-Dichloro-3-Methylthiophene definitely claims a spot in this category. The journey of thiophene derivatives dates back to the surge of heterocyclic chemistry in the 19th century, spurred by research into coal tar compositions. For 2.5-Dichloro-3-Methylthiophene, industrial interest picked up in the late twentieth century, mostly propelled by a rapid demand for custom building blocks during the synthetic drug boom. Academics and process chemists searching for new halogenated and methylated thiophenes found that tweaking the 2 and 5 positions on the ring amplified chemical versatility, and after rigorous method development, large-scale production got underway. As regulatory needs around pharmaceutical intermediates expanded, detailed data sheets started to appear and safety protocols tightened, making this compound far more accessible than it once was.
At its core, 2.5-Dichloro-3-Methylthiophene slots in among the more functionalized thiophenes, favored for its ability to introduce both electron-rich and electron-deficient behavior in synthetic projects. Bulk suppliers carry it for use as a key ingredient in preparing advanced intermediates, specialty polymers, and agrochemical scaffolds. For a lot of bench chemists, having the right halogen in the right place can act as a chemical switch, influencing everything from solubility to biological activity. Its distinctive odor and oily consistency mean you don't forget your last encounter with a bottle in the lab. Chemists turn to it during the lead optimization phase, especially where adjusting lipophilicity or metabolic stability takes priority.
Most bottles of 2.5-Dichloro-3-Methylthiophene reveal a colorless to pale yellow liquid with a sharp, pungent smell that’s hard to miss. The molecular formula clocks in at C5H4Cl2S, and the molecular weight stands at 183.06 g/mol. Boiling point hovers around 187–189°C, making it workable in a standard fume hood but demanding careful monitoring during evaporative steps. It dissolves in organic solvents like dichloromethane, ethyl acetate, and tetrahydrofuran, and resists water, thanks to the hydrophobic thiophene core. The pair of chlorines and the lone methyl group decorate the five-membered ring, providing just enough reactivity for electrophilic and nucleophilic substitution, but unstable enough that you tend to work quickly and keep waste tightly sealed.
Producers ship 2.5-Dichloro-3-Methylthiophene under strict chemical grade specifications, aiming for purity above 97% by gas chromatography. Standard packaging uses amber vials or aluminum containers to guard against light-induced degradation, and the hazard label clearly flags both irritant and environmental risk symbols. The chemical abstract service (CAS) number—67786-25-8—accompanies shipments, and every lot comes with a certificate of analysis noting water content, residual solvent traces, and sometimes even batch-level melting or boiling points. Barcode labels and digital tracking are now standard, thanks to regulatory pushes in drug precursor logistics, so anyone ordering the compound for an industrial project can pull up compliance data back to the source.
Lab synthesis of 2.5-Dichloro-3-Methylthiophene starts by chlorinating 3-methylthiophene using thionyl chloride or N-chlorosuccinimide under controlled temperatures. The challenge here involves hitting the right regioselectivity, as uncontrolled chlorination can scatter the halogens around the ring or over-minimize the yield. Process optimization relies heavily on solvent choice and careful adjustment of stoichiometry, since too much chlorination feeds the formation of trichloro variants, while too little might leave the starting material untouched. Once the reaction wraps up, distillation strips out solvents and side-products, leaving the distinctive pale oil behind. Large manufacturers have honed this to reduce waste, sometimes recycling unreacted chlorinating agents—good for both yield and environmental management.
This molecule’s real charm shows up in further transformations. Its two chloro groups open the door for metal-catalyzed couplings, such as Suzuki or Stille reactions, where the positions 2 and 5 serve as launching points for aryl or alkyl attachments. The methyl group gives some electron-donating push, making selective functionalization at adjacent positions more achievable. It plays well in Friedel-Crafts alkylations, and switching the halides out with more exotic nucleophiles can produce libraries of new analogs—a regular routine for drug discovery teams hunting for new potent scaffolds. Synthetic strategies often capitalize on the ready availability of this derivative, and chemists value its ability to act as a flexible intermediate rather than a final target molecule.
Depending on whose catalog you're browsing, you might see 2,5-dichloro-3-methylthiophene listed as: 2,5-Dichloro-3-methyl-thiophene, 3-Methyl-2,5-dichlorothiophene, or even DCMT in shorter lab slang. Trade suppliers sometimes use abbreviated names for ease in inventory control, which keeps tedious chemical nomenclature off the order forms. On patent filings and research articles, the fully systematic name appears, eliminating ambiguity. By whichever title, researchers learn to check structural diagrams—misreading a supplier’s number for a different dichlorothiophene is a mistake most chemists only make once.
Working with halogenated thiophenes always demands respect for gloves, goggles, and good airflow. 2.5-Dichloro-3-Methylthiophene gives off vapors that irritate eyes, nose, and skin, often prompting labs to keep bench stocks tightly capped and extra packs of nitrile gloves close by. Spills produce an odor that lingers for days, making accurate pipetting and quick cleanup a matter of practice more than theory. Waste often lands in halogenated organics drums, fitting strict chemical disposal rules. As with most small, dense organosulfur compounds, fire risk rises if storage guidelines go ignored. Out in the field, suppliers train bulk handlers on spill protocols and proper disposal, keeping team safety front and center.
Pharma and agrochemical teams use 2.5-Dichloro-3-Methylthiophene in early-stage molecule synthesis, seeking structures with antifungal, antiviral, or herbicidal traits. Polymer chemists sometimes incorporate this thiophene ring to tweak conductivity or stability in advanced materials. Flavor and fragrance creators, though cautious about its strong sulfur notes, have run exploratory work into aromatic derivatives—not for the final product, but as stepping stones to more subtle compounds. Some teams in materials science also use functionalized thiophenes to build blocks of OLEDs and organic transistors, leveraging the chemical’s reactivity without relying on it for bulk structural work. Research shows a larger share of future patents referencing this synthetic intermediate in connection with new bioactive agents or smart materials, underscoring its versatility.
Each year, new academic papers present creative ways to convert 2.5-Dichloro-3-Methylthiophene into more complex molecules, often pushing for greener processes or more selective couplings. Green chemistry advocates tune reaction profiles—switching from toxic solvents to bio-based alternatives, or designing catalysts that work at room temperature. Labs in medicinal chemistry explore fresh analogs around the thiophene ring, figuring out which substitution patterns offer antimicrobial or anti-inflammatory effects. Meanwhile, industrial teams focus on scaling cleanly: reducing impurity profiles, lowering the carbon footprint, reusing solvents, and automating tricky steps for batch-to-batch reliability. These incremental improvements mean the molecule becomes safer to make and to work with, easing its path into regulatory clearance for new projects.
Tests reveal that 2.5-Dichloro-3-Methylthiophene, like many organosulfur compounds, can irritate mucous membranes and act as a skin sensitizer after repeated exposure. Chronic toxicity studies sit a bit thin on the ground, but related thiophene derivatives—particularly those with multiple halogens—have drawn closer environmental scrutiny for persistence and potential bioaccumulation. Inhalation produces coughing and sometimes headaches, an experience firsthand in graduate labs where fume extraction didn’t quite cover the whole bench. Waterways remain a concern for disposal, so best practices route all liquid waste for incineration rather than drain disposal. Ongoing studies aim to clarify long-term risk profiles, especially in large-scale manufacturing or open-environment handling scenarios.
A lot of forecasting in synthetic chemistry calls for more modular intermediates, and 2.5-Dichloro-3-Methylthiophene checks that box. As demand rises for pharmaceuticals and specialty chemicals with tighter purity specs, intermediates that are both reactive and reliably made will stay in demand. Advances in continuous-flow chemistry, already common in pharma manufacturing, open paths to faster, cleaner chlorination and less hazardous waste, which appeals to both economic and environmental interest. There's growing conversation about bio-based synthesis routes that start from natural thiophene sources, though practical, price-point breakthroughs remain down the road. With each leap in catalytic techniques, and smarter digital tracking for shipments, this methylated, dichlorinated thiophene seems set to stick around as both a workhorse and a test-bed for what greener, more efficient chemistry can do.
2.5-Dichloro-3-Methylthiophene sports a chemical formula of C5H4Cl2S. Count up five carbons, four hydrogens, two chlorines, and a sulfur — that’s what you’ve got in each molecule. The molecular weight lands around 183.06 grams per mole. These aren’t just numbers for a lab notebook. They show the hand chemists play with. From experience, even small tweaks like another methyl group or one less chlorine can tip the balance when you’re designing or tweaking new materials.
The formula matters because it carries clues about what this compound can do and where trouble might turn up. Chlorines locked onto a thiophene ring change its reactivity, making this molecule a solid candidate for building more complex chemicals. The molecular weight isn’t just for calculations or shipping paperwork. Labs must know it to set up reactions, estimate dosages, or guess how easily this stuff will drift through the air or stick to a surface. Think of how paint smells — smaller molecules spread out fast. A chunkier weight sometimes means a material holds steady and won’t flash off into the air.
Consider the uses out in the world — not just in textbooks. Modified thiophenes, like this one, feed into making specialty polymers, pharmaceuticals, or even bits of electronics such as organic solar cells. The methyl and chloro groups push the electronic properties into unique territory, making these compounds valuable to folks who want stable, tunable materials. One time, while working on conductive polymer research, a similar substituted thiophene rescued the day by offering just the right mix of stability and flexibility.
But with value comes a challenge. Chlorinated aromatics get flagged by health and safety crews. They linger, sometimes stubbornly breaking down in the environment. The weight and structure hint at risk as well as reward. Responsible labs don’t just synthesize and forget — they track, neutralize, and plan for what happens once a beaker empties.
Nobody likes being caught by surprise, especially in the lab. Tools like molecular weight calculators and predictive software save time, but hands-on safety matters more. The more unique compounds with halogens or sulfur cycle through labs, the more pressure grows to tighten up storage, ventilation, and disposal. One recurring headache involves dealing with waste — not just because local rules say so, but out of respect for anyone downstream who drinks the river water or works on the same bench tomorrow.
Looking for tweaks — swapping out chlorines, tweaking methyl placements, or picking different ring systems — these aren’t just academic games. Sometimes new structures deliver the needed performance with less environmental or health baggage. Crossing paths with green chemistry approaches felt awkward at first, but the logic clicks. Cut hazards, still solve problems, sleep better at night.
Raw data like a chemical formula or weight kicks off conversations that lead to better science. The real work shows up in how chemists use these building blocks, adapt safer habits, and weigh short-term wins against long-term costs. Everyone from students to seasoned hands stands to gain by asking a bit more about what a string of numbers and symbols really means.
Ask anyone who has spent time in a chemical lab about the importance of specialized starting materials, and you’ll probably hear a story about how the success of a whole project hung on finding the right compound. That’s how I ended up reading a lot about 2.5-Dichloro-3-Methylthiophene. The name sounds pretty intimidating, but at its core, it’s a ring-shaped molecule with chlorine and methyl tweaks that help it slot into a lot of different places in chemical reactions.
Folks in the pharmaceutical industry look for structures like this when developing new drugs. Aromatic rings with sulfur and halogen groups may seem like fine print on a patent, but in practice, these features can mean big changes in a medicine’s behavior inside the human body. My own work with medical chemists taught me that new antibiotics and antifungals, especially, get built step-by-step with building blocks like this molecule. It’s not doing the healing itself, but it’s laying down the path for something life-saving down the line.
In agri-tech, 2.5-Dichloro-3-Methylthiophene gets attention for similar reasons. Farmers expect modern pesticides to be selective and powerful. Companies respond by blending together new combinations of chemical rings, searching for that balance between effectiveness and low environmental impact. This compound’s reactive sites let it snap into new pesticide formulas, a bit like snapping LEGO bricks together to get a new figure. After seasons of volunteer work on small-scale organic farms, I’ve seen how plant health ties in closely with the chemistry that shapes pest management ideas coming out of industrial labs.
Move past medicine and crops, and you’ll find 2.5-Dichloro-3-Methylthiophene turning up in surprising spots—like electronic components. Polymers based on thiophene rings help improve things like display screens, solar panels, and sensors. The way electrons zip across these rings affects performance and flexibility, so introducing chlorine and methyl groups isn’t an afterthought; it’s a design choice. As more devices need to flex, bend, or endure heat, you can bet researchers will keep eyeing this molecule for the electrical punch it packs when placed just right in a panel or a sensor.
Chemistry rarely solves all its problems for good. Stories I’ve heard from industrial safety officers tell me that careful handling of chlorinated compounds matters, both for workers and the environment. It pushes chemists to keep one eye on how these pieces are made and broken down. Working toward greener synthesis and better recycling options for thiophene derivatives should be part of the conversation in labs and factories, not an afterthought. The health and safety toolbox—good ventilation, gloves, process checks—makes a difference, but so does smart design that thinks beyond a single use or product.
Across pharma, farming, and electronics, 2.5-Dichloro-3-Methylthiophene doesn’t get attention like finished products do. Still, the research around it highlights a network of specialists, from bench chemists to engineers to safety teams, all nudging each other to create safer, better, and more useful materials. The best advice I ever received in chemical research was to keep listening, because even subtle changes in a molecule can lead to much bigger leaps in the world outside the lab.
Chemicals like 2.5-Dichloro-3-Methylthiophene attract attention for their role in research and manufacturing, but day-to-day realities often start in stockrooms and storerooms. I remember the sharp, ticklish citrus-like tang in the air the first time I opened a drum of this compound for a university experiment. That experience stuck—chemicals with strong odors or reactivity have a way of making lasting impressions and reminders about safety. Proper handling isn’t just a set of rules; it’s about healthy lungs, clear skin, and a clean lab.
This compound won’t usually explode or burst into flames at room temperature, but it brings its own set of risks. It reacts with strong oxidizing agents and acids, so crews keep it far away from anything with “peroxide” or “nitrate” on the label. I once saw a shelf clouded and crusty from a mistake in chemical storage; one misplaced solvent bottle near a reactive compound set off a chain of corrosion that chewed through the metal. Chemical cabinets built from treated, coated steel or sturdy polymer give peace of mind. Labels should be bold, easy to read, and checked regularly.
Cool, well-ventilated rooms work best. Warm spots ramp up reaction rates and evaporation, which means more fumes and potential fire risk. I’ve cracked a window in January for these sorts of jobs—chilly fingers beat a coughing fit. Store containers away from heat sources, direct sunlight, and high humidity, since excess moisture (even in the air) can lead to slow breakdown and unwanted side products.
It’s tough to keep a workspace tidy, but loose caps or half-sealed jugs invite trouble. Every time I’ve seen a container with dried rings or discolored threads, I hear the voice of my old supervisor: “If you can smell it, you’re being exposed to it, and so is your neighbor.” Secure all lids after use, and transfer in small quantities whenever possible—smaller spills mean easier cleanups. Larger drums should stay on pallets so they avoid contact with puddles or direct floor grime.
Material safety data sheets help, but the human factor counts. I always keep gloves, splash-proof goggles, and coat at hand, even if the procedure “should go quickly.” Skin contact can cause irritation; inhaling the vapor over and over brings headaches and worse. Involving your team in regular reviews of storage space reduces missed steps. Spillage absorbs best with inert materials—think clay or sand, not sawdust or paper towels.
Simple practices save the most trouble. Once a week, a careful walk through the chemical storage area finds early signs of leaks or damaged bottles. Good lighting highlights drips and crusts. Ventilation fans should hum along; if the air grows sour or sharp, it’s time to crack the windows wider and check vents for dust bunnies or blockages.
Disposal builds up behind closed doors, with leftover dribbles of chemicals and crusts in vials. Left too long, those little leftovers become the next lab accident. Certified waste disposal outfits haul away the dangerous stuff. Keeping logs of who used what and when can put a stop to confusion or cross-contamination.
Storing and handling 2.5-Dichloro-3-Methylthiophene goes far beyond the textbook. Even after years in labs, every new shipment prompts a double-check of the usual routine. Proper ventilation, sturdy shelves, clear protective gear, and well-marked containers—nothing replaces these basics, and they aren’t complicated. Treating every chemical, not just the notorious ones, as a potential hazard builds habits that protect people and property.
Chemicals like 2.5-Dichloro-3-Methylthiophene turn up in research labs and the chemical industry fairly often. People who work with specialty chemicals know the importance of learning about hazards up front. In this case, we’re looking at a compound with a strong, sometimes unpleasant odor. It doesn’t show up on a grocery list, and most folks outside chemistry circles may never hear about it. Still, the risks that come with handling these compounds don’t care about your job title—skin, lungs, and the workplace environment all stand to suffer from bad handling.
Every chemist I’ve met has a strong respect for anything carrying the “dichloro” label. Chlorinated organics like this one can irritate the eyes, skin, and respiratory tract. Coming in contact with a splash, even a small one, has left plenty of folks feeling the burn—sometimes literally. Vapors from volatile organosulfur compounds sting the nose and eyes pretty fast, reminding you not to get casual in the lab.
On top of discomfort, there’s the risk of more serious long-term health issues. Breathing in chemical vapors repeatedly over weeks or months can create respiratory problems. People who handle these kinds of chemicals every day owe it to themselves and their coworkers to treat the stuff with respect. Data sheets for 2.5-Dichloro-3-Methylthiophene call out its toxic and irritating nature. Chronic exposure could raise cancer risks, especially if warning signs get ignored.
In the years I worked in university research settings, we took safety seriously. Yet I saw colleagues cut corners—removing gloves “just for a moment,” or lifting their masks for a quick question. These are the moments that got people into trouble. I remember seeing a lab mate suffer a nasty skin rash from missing a dot of liquid when pouring. Another time, strong vapors sent an experienced technician out for fresh air fast, coughing and rubbing his eyes.
No matter how routine the day feels, chemicals like 2.5-Dichloro-3-Methylthiophene don’t reward shortcuts. Even small lapses lead to burns, rashes, or feeling light-headed. I found that reminding myself of these stories, not just reading labels, helped keep risk front and center.
Chemical risk seems abstract—until it lands on your skin. Gloves, goggles, and a good fitted mask protect against splashes and vapors. I’ve learned not to trust old gloves; if they look worn, swap for new ones. Fume hoods do more than cut smells—they grab harmful vapors before anyone has to breathe them in. Working outside the hood, even for a few minutes, isn’t worth the risk.
Spill kits and eye-wash stations deserve respect. In a crunch, they can mean the difference between a rough story and lasting damage. Everyone deserves a quick response when things go sideways. People in shared spaces should make it a routine to double-check storage. 2.5-Dichloro-3-Methylthiophene should stay in tightly sealed containers, kept away from direct sunlight and heat. A little coordination—to label bottles, to note quantities—keeps accidents at bay.
Some labs now look for less hazardous alternatives or invest in closed-system handling where possible. Tech advances make exposure less likely, but they don’t turn chemicals into harmless water. Training keeps safety from fading into the background. Open conversations about near-misses, smart gear, and why it all matters help new folks avoid the mistakes of those who came before. Trust between coworkers, not just regulations, proves most valuable.
So the take-home lesson is this: respect the substance, trust your senses, and never let haste outweigh caution. Safety comes from people as much as protocols.
Those who’ve worked with fine chemicals know what a difference purity can make, especially with compounds like 2.5-Dichloro-3-Methylthiophene. Labs, pharma companies, and research folks usually look for this compound at purity levels of at least 97%. Anything below that can lead to unpredictable results or more byproducts during syntheses. Most reputable suppliers push the purity to 98% or even 99% for sensitive uses, since even a tiny contaminant can derail a reaction. I’ve watched projects drag out for weeks only to trace the problem back to a slightly dirty starting material — a frustrating experience for anyone on deadline.
Quality control relies on advanced chromatographic techniques like HPLC and GC to back up those purity claims. Trustworthy vendors also supply a certificate of analysis (COA) so nobody is guessing what’s in the bottle. These certificates give buyers the confidence to proceed, especially when their own processes hinge on perfect starting materials.
2.5-Dichloro-3-Methylthiophene isn’t a bulk commodity, so packaging tends to be a bit more specialized. For small-scale research, glass bottles of 5g, 10g, 25g, and 100g show up most often. Polyethylene or PTFE-lined caps keep any chance of leaks or contamination down. Glass beats plastic here, mainly because the compound needs protection against air and moisture. Companies thinking about bigger runs — custom synthesis, pilot batches, or manufacturing — often request 500g, 1kg, or even 5kg containers. That typically means sealed aluminum bottles or HDPE drums, clearly labeled and shrink-wrapped to satisfy safety regulations.
Shipping rules can complicate things. Because 2.5-Dichloro-3-Methylthiophene carries some hazard labels, packaging has to meet regulatory standards for hazardous materials. This isn’t up for debate — skip a step and the shipment gets stuck or sent back, wasting everyone’s time.
A lot can go wrong during storage. From experience, leaving a bottle open on the benchtop ends with degraded or contaminated product, which then throws off yields and purity in anything down the line. Strong, resealable caps and clear labeling are small things, but they save hassle later. Suppliers who pack in a nitrogen atmosphere or vacuum-seal containers earn extra trust — oxygen is a sworn enemy for many sulfur-containing aromatics like this one.
Temperature shouldn’t be ignored either. Even if 2.5-Dichloro-3-Methylthiophene isn’t especially volatile, a cool and dry setting prolongs its life and keeps reactions consistent. Labs that invest in a chemical refrigerator or regulated cabinet end up with far fewer surprises.
Supply chain disruptions do happen, and I’ve received different batches with slightly different container sizes or label formats. A move toward more consistent packaging options across suppliers would help chemists plan ahead, especially those managing inventory for big projects. Reusable and recyclable packaging would also cut down on lab waste — something every research group faces during routine runs.
Better online documentation helps bring everyone onto the same page. Buyers shouldn’t need to dig deep for storage and handling tips or hazard info. Improving supplier transparency around these details would lighten the load for new chemists learning the ropes.
2.5-Dichloro-3-Methylthiophene might seem like just another building block, but experience shows details matter. High purity, tough packaging, good labels, and easy-to-access support add up to smoother workdays and better results on the bench. Reliable partners take care over each step, which lets everyone else focus on the real chemistry.
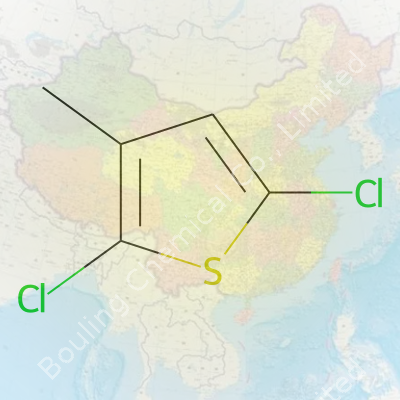

| Names | |
| Preferred IUPAC name | 2,5-dichloro-3-methylthiophene |
| Other names |
3-Methyl-2,5-dichlorothiophene 2,5-Dichloro-3-methylthiophene 2,5-dichloro-3-methyl-thiophene |
| Pronunciation | /ˈtuː pɔɪnt ˈfaɪv daɪˈklɔːroʊ ˈθriː ˈmɛθɪl θaɪˈoʊfiːn/ |
| Identifiers | |
| CAS Number | [19311-91-8] |
| 3D model (JSmol) | `3DView: C1=C(SC=C1Cl)CCl` |
| Beilstein Reference | 0421846 |
| ChEBI | CHEBI:137009 |
| ChEMBL | CHEMBL3182502 |
| ChemSpider | 186491 |
| DrugBank | DB08324 |
| ECHA InfoCard | 19d4aa64-023c-4630-9869-499c8fc9d9a9 |
| EC Number | 6290-99-7 |
| Gmelin Reference | 70761 |
| KEGG | C19283 |
| MeSH | D017979 |
| PubChem CID | 13611277 |
| RTECS number | XZ2060000 |
| UNII | MA24B6Q0X7 |
| UN number | UN3345 |
| CompTox Dashboard (EPA) | DTXSID70110343 |
| Properties | |
| Chemical formula | C5H4Cl2S |
| Molar mass | 163.07 g/mol |
| Appearance | Colorless to light yellow liquid |
| Odor | Strong odor |
| Density | 1.44 g/mL at 25 °C (lit.) |
| Solubility in water | Insoluble |
| log P | 2.98 |
| Vapor pressure | 0.0957 mmHg at 25 °C |
| Acidity (pKa) | pKa = 0.84 |
| Magnetic susceptibility (χ) | -0.00012 |
| Refractive index (nD) | 1.562 |
| Viscosity | Viscous liquid |
| Dipole moment | 1.76 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 289.2 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -5726.8 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. |
| GHS labelling | GHS07; GHS09; Warning; H302; H315; H317; H319; H410 |
| Pictograms | GHS05, GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P273, P280, P301+P312, P304+P340, P305+P351+P338, P312, P330, P337+P313, P362+P364, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) | 2-3-0 |
| Flash point | 61°C (closed cup) |
| Lethal dose or concentration | LD50 oral rat 1800 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 > 2000 mg/kg |
| NIOSH | SN1225000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 5g |
| Related compounds | |
| Related compounds |
Thiophene 2,5-Dichlorothiophene 3-Methylthiophene 2-Chlorothiophene 3-Chlorothiophene |