Back in the heyday of the 20th-century chemical revolution, labs worldwide started to map out ways to take basic aromatic rings and make something new—something that could form the backbone of more complex molecules. Chemists searched for functionalized thiophenes because these opened doors to pharmaceuticals, advanced materials, and specialty dyes. Out of all the variants, 2,5-dibromothiophene grabbed attention. Research in the late 1960s and 1970s, especially from European and Japanese teams, outlined early routes and uses. These discoveries came not from modern computers, but from hands-on bench work and trial and error.
2,5-Dibromothiophene has become a staple for anyone working in organic synthesis. The two bromine atoms on the ring make it reactive yet stable enough to store in bottles on a lab shelf. It’s not an everyday chemical for the public, but it stays in steady demand with every custom synthesis project, especially in R&D departments focused on electronics, pharmaceuticals, and specialty polymers.
Pick up a vial, and you’ll see a colorless to pale yellow liquid, sometimes a low-melting solid depending on storage and purity. The melting point usually sits around 20°C, and it boils near 220°C. The smell, typical for brominated aromatics, lingers a bit, a sign of latent volatility. The presence of two bromine atoms, both on the thiophene’s even-numbered positions, makes it more dense and heavier than the plain thiophene ring. It dissolves readily in most organic solvents, but keeps its distance from water. In my own time with it, gloves are a must—the stuff tingles on unprotected skin, and brominated molecules usually spell trouble in terms of long-term exposure.
Typical containers arrive labeled with CAS number 3141-27-3, and purity sits at a solid 97% or more for most commercial sources. As a regulated chemical, labels carry hazard icons signaling irritant or toxic hazards. UN shipping numbers and GHS codes get checked closely in shipping logs. My own shipments have always packed a distinct chemical whiff. Refrigerated storage helps keep the stuff settled and ready for the next synthetic run.
Making this compound often centers on brominating regular thiophene. Dropwise addition of bromine with chilling, and you get stepwise dibromination. Most protocols control the substitution carefully so both bromines hit the 2- and 5-positions. Industrial-scale manufacturing tunes this with catalysts and solvents, sometimes recycling bromine for cost reasons. On a smaller scale, glassware and slow addition are enough, though vigilant ventilation is non-negotiable. Running these reactions brings back memories—the red vapor cloud from overenthusiastic bromine additions, the slow crystallization on a chilly window ledge.
Two bromines on the ring make this compound a workhorse for cross-coupling reactions. Suzuki and Stille couplings leap to mind—pop one, then both, bromines off and attach anything from alkyl groups to aryl rings. This opens new branches on polymers, pushes forward pharmaceutical scaffolds, and builds up more complicated molecular electronics. Each substitution step has a knack for delivering surprises—sometimes yields dip or regioisomer complications arise. Dehalogenation, Grignard reactions, and metalation broaden the scope even more, each with its quirks and learning curves.
Chemical catalogs keep it straightforward—"2,5-Dibromothiophene" stands as the go-to name. Lists sometimes call it "Thiophene, 2,5-dibromo-", or "2,5-Bis(bromo)thiophene". The CAS number locks it down universally, so mistakes don’t creep in between orders or paperwork. Brands also assign catalog numbers for easier tracking, but among researchers, the base name gets the job done without any confusion.
Despite its valuable chemistry, nobody describes handling 2,5-dibromothiophene as risk-free. Skin and eye irritation turn up fast on accidental contact. Inhaled vapors light up mucous membranes, and chronic exposure to brominated compounds raises red flags for long-term health effects. In the labs where I’ve worked, full PPE and chemical hoods were a given—no shortcuts. Waste solutions need segregating in halogenated waste cans, following protocols that keep local water tables free from halide pollution. Spill kits always wait nearby, and broken bottles mean an immediate halt for cleanup.
Most activity circles back to research synthesis. In electronics, scientists use this compound as a precursor for conductive polymers—think poly(thiophene) derivatives essential to flexible display screens and sensors. Drug researchers tap it to form rare heterocyclic compounds, tinkering with bromine swaps to test new biological effects. Specialty materials feature it as a stepping stone, whether for new light-absorptive layers in solar energy or fresh synthetic dyes. Each new field seems to cook up new demand for the molecule, and every block in the molecule’s structure opens a fresh avenue for investigation.
Chemists treat 2,5-dibromothiophene as a versatile switchboard for organic innovation. Ongoing projects in academia and industry keep finding new tweaks using this ring—adding solubilizing groups for printable electronics, chaining together rings for organic LEDs, and testing novel drug candidates with the thiophene core. Tinkering with its chemistry sharpens skills on modern cross-coupling techniques, and published literature serves as proof that creative molecular design still relies on workhorse intermediates like this one. In my lab days, troubleshooting Suzuki reactions or scaling up syntheses always felt like a blend of scientific rigor and creative improvisation.
Long-term safety data lags behind the rapid pace of research. Animal studies and workplace reports warn that extended exposure can lead to organ damage, particularly to liver and kidneys. Acute contact typically brings on irritation, but a few papers raise alarms about more serious effects from repeated dosing. Agencies such as the EPA and ECHA in the EU continue to update their risk evaluations. Every new regulation nudges companies to step up protection, drive green chemistry improvements, and substitute less hazardous routes when possible. Until more definitive long-term toxicity data emerges, workers rely on good sense, careful logs, and the best available PPE.
Looking ahead, demand will likely track the rise of organic electronics, next-generation pharmaceuticals, and sustainable materials. Researchers continue searching for halogen-free or less toxic alternatives, but so far, nothing quite replaces the unique reactivity and reliability of 2,5-dibromothiophene in high-value syntheses. Innovations may soon land on greener synthetic pathways, higher selectivity, and more robust safety practices. From my own experience in the lab and reading the literature, new discoveries often circle back to trusted intermediates, and this molecule holds steady as a trusted if challenging partner in chemical invention.
2,5-Dibromothiophene carries the formula C4H2Br2S. That boils down to a thiophene ring—four carbons, one sulfur—decked out with two bromine atoms. Both bromines park themselves at the 2 and 5 positions of the five-membered ring. Chemists appreciate this molecule because it’s simple, but it holds a key spot on the path to more advanced organic compounds.
Pull up the structure and two things stand out. There’s a flat, five-membered thiophene ring, with sulfur nestling between two pairs of carbons. Bromines latch onto carbons next to sulfur—the 2 and 5 slots. The symmetry brings a certain elegance. It isn’t just for show: The reactivity and usefulness of this molecule grow from these bromine anchors and the stable ring. Not every molecule’s design lands this kind of functional balance.
In my work with organic synthesis, it’s clear why chemists keep coming back to dibromo derivatives like this one. Those bromine atoms do more than just decorate the ring. They act as perfect placeholders for swapping in new chemical groups. This swappability gets a lot of attention in creating complex molecules, especially for pharmaceuticals or advanced conjugated materials. Fact is, simple substitutions here let you build truly elaborate molecular machines.
A closer look at 2,5-Dibromothiophene’s practical side shows its popularity in making building blocks for semiconductors, sensors, and organic LEDs. The electronics world leans on molecules like this because they combine stability and reactivity in equal measure. Researchers published in journals like Advanced Materials and Organic Electronics describe using this compound as a backbone for materials carrying electricity in flexible displays and solar cells.
A 2018 review in Chemical Reviews brought up the big role thiophene derivatives play in tuning the performance of organic electronics. The two bromines are perfect for launching cross-coupling reactions—the go-to step to create larger conductive polymers. That’s an edge you don’t get with less reactive halides or unsubstituted rings.
Brominated molecules aren’t completely rosy. Manufacturing brings up concerns around waste and safety. Bromine compounds sometimes pose toxicity and disposal challenges. I’ve seen this firsthand in labs with strict handling and waste disposal rules that stretch timelines and raise costs.
There’s some hope in greener chemistry approaches. Switching to less hazardous solvents, capturing byproducts for recycling, and tuning reaction conditions can cut down on the environmental impact. Efforts like these already reduced the footprint for similar halogenated organics in industry. Some groups turn to direct functionalization of thiophene itself, which slashes the need for repeat halogenation steps and trims away excess waste.
Diving into synthesis requires a few key choices, and 2,5-Dibromothiophene often stands right at the crossroads. Its physical structure isn’t just a chemical curiosity—it’s a springboard. The way those bromines cling to the ring means you can grab hold, break a bond, and build out. What grows from this simple molecule powers long-life organic circuits, light-up displays, and a whole class of modern electronic parts.
Spend a few years around a chemistry lab, and odd little compounds become familiar faces. 2,5-Dibromothiophene is one of those—a molecule that looks simple, but plays a big role across the world of advanced materials and research. At its core, it’s a thiophene ring with two bromine atoms locked at positions that just happen to be convenient for chemists searching for something they can tweak or build on. You’ll most often spot it not on its own, but as a building block for something with bigger ambitions.
People who follow electronics often imagine hard, shiny chips, or maybe some flexible display in a gadget. There’s a whole other world, though, where scientists try to build circuits with organic molecules. 2,5-Dibromothiophene helps make that possible. Chemists reach for it when they want to create new polymers—especially conductive ones, like polythiophenes. These materials show up in things like organic solar cells, light-emitting diodes (the OLEDs in fancy TVs), or transistors that could end up making electronics lighter and bendier.
From what I’ve seen, the trick is in how those bromine atoms make handling thiophene molecules easier during synthesis. You can swap them out, link them up, or guide reactions toward long chains. Its structure simplifies the work needed to pull off Suzuki coupling and Stille reactions—two tools that allow scientists to stitch together complex molecules piece by piece. Just in the last decade, research has shown how 2,5-Dibromothiophene can open doors to organic electronics that work better and last longer.
Back in grad school, I remember how many specialty molecules started from simple ring compounds like this one. 2,5-Dibromothiophene finds itself drafted as a starting material for more advanced chemicals in pharma labs. Medicinal chemists, chasing new drug candidates, will use it to build sulfur-containing molecules. These molecules behave a little differently, and sometimes that difference makes all the difference in targeting cancer cells or trying to quiet overactive immune systems.
Beyond drugs, there’s a whole category of specialty chemicals that rely on this brominated thiophene. Dyes, pigments, and advanced intermediates all stem from pieces like this. I once worked on a project focused on specialty coatings, where tweaking the backbone of the polymer—even by just adding a sulfur atom—could shift color, resilience, or how something fends off moisture. Dibrominated thiophenes popped up there too, because small changes upstream could ripple through the final product.
What stands out to me about 2,5-Dibromothiophene is how flexible it proves in the hands of a good chemist. Universities and startups keep looking for ways to use it in sensors, organic field-effect transistors, and even in experimental batteries and supercapacitors. The chemistry is all about the carbon-sulfur backbone, which lends stability and electronic properties that silicon just can’t match.
Access to affordable and reliable supplies still shapes what researchers can do. For scientists working outside big-budget labs, cost and purity hang over every project. Some green chemistry efforts look for ways to synthesize it with less waste or lower environmental impact, since brominated compounds involve some rough manufacturing steps. Pushing for better recycling, cleaner processes, and smarter uses are challenges this field faces as research keeps building.
Years ago, in a cramped university lab, a colleague handed me a vial of 2,5-dibromothiophene. Before I checked the paperwork, I inspected the liquid. The eyes always make the call before instruments do. For this chemical, a slightly yellow tint, sometimes clear depending on supply, tells you much about what might be lurking inside. Pure 2,5-dibromothiophene usually comes clear to pale yellow in color, a sign that big contaminants haven’t snuck into the batch during synthesis or storage.
Seeing a darker shade, or anything cloudy, hints there’s trouble. I’ve unpacked shipments where oxidation during storage led to off-putting amber hues. Once, a faint earthy smell mixed with what can only be described as electrical insulation told the story better than the certificate of analysis. Color and transparency remain the quickest indicators; chemists around the world still lean on sight before equipment.
Any supplier pushing material above 98% purity wants you to believe the number means worry-free experiments, but there’s room for skepticism. High purity must translate in practice, not just on paper. Impurities, even in the low percent range, react unpredictably. Syntheses involving arylation or Suzuki coupling have failed on me because of halide or organic residues sitting at half a percent.
Suppliers aiming higher, to 99% or better, tend to rely on chromatographic methods and strict quality control. I’ve found that sourcing from reputable labs who invite questions, disclose their cleaning and distillation steps, and disclose batch-level analysis gives a better shot at consistency. Some distributors frustrate their clients by obscuring test details. You want HPLC or GC results, not vague claims. I once trusted a sample labeled 98% which ruined my reference yields in a Pd-catalyzed reaction. It turned out to be just shy of 97%, but the wrong 1% gave headaches that cost me a week.
Working in a synthesis lab, a little contamination isn’t theoretical. Halogenated thiophenes like this pick up water and react with ambient oxygen. Over time, even tiny levels of moisture or oxygen encourage decomposition to dibromobutenes or sulfur compounds – and these don’t just lower your yields. They produce misleading data downstream, inflate costs, waste time, and sometimes, if safety checks get lazy, spark toxic gas production.
Labs seeking better results need suppliers who ship chemicals in airtight amber glass bottles, clearly labeled with storage instructions and batch numbers. Giving end-users direct access to lot-specific chromatograms and spectra also builds trust. In settings where budgets feel tighter every year, supporting longer shelf life with minimal degradation should stand at the top of everyone’s list.
CAT scans for your chemistry: that’s how I think of proper purity information. Without it, even the best experiment plans stumble. Whether running benchwork for drug development or chasing cleaner optoelectronic materials, the quality of 2,5-dibromothiophene you start with either paves the road or fills it with potholes.
Genuine progress depends on supplier transparency, smarter storage, and constant communication between lab and distributor. I take time now to validate every new batch myself. It creates extra work but pays off by preventing much bigger headaches. Choosing suppliers based on detailed analysis and real-world feedback works far better than rolling the dice with a nameless bulk bottle.
I’ve spent time around chemical labs, and the truth is, words on a label never quite prepare you for a leaky bottle or a loose cap. 2,5-Dibromothiophene has a pretty specific smell, and for good reason—it’s no friend to your nose or lungs. Mishaps can turn serious fast. It’s not just about cleanup; it’s about long-term health and, sometimes, the safety of everyone in the building.
Shelves stacked with bottles tempt shortcuts, but storing this compound demands care. I avoid sunlight like the plague with anything ‘dibromo’—heat and light invite breakdown, which means hazardous surprises. So, I always pick a cool, dry spot, somewhere ventilation keeps air fresh, but not a whiff of chemical scent escapes. Sturdy cabinets designed for corrosives or flammables keep things contained. No cardboard boxes tucked in dark corners—the containers need to stay upright and secure, lids sealed tight.
Clear labels save headaches. Two weeks into a project, nobody remembers which bottle came from which order without readable names and dates. Labels in bold marker, hazard symbols front and center, and records stored nearby help everyone keep track on busy days. It takes discipline, but sloppiness here has cost careers and, in rarer cases, lives.
Too many people skip gloves “just this time.” I’d never risk that. Nitrile gloves offer a good barrier, and I check them for holes before doing anything. Goggles that hug my face—my eyes still thank me. Lab coats and long sleeves form a last line of defense against splashes. Years ago, a careless reach led to a ruined shirt, but nothing got through thanks to the extra layer.
I treat transfers with caution. Small beaker, slow pour, and always over a spill tray. Ventilation is a priority—I work near a fume hood or at least with a fan blowing toward an open window. Splashing a few milliliters on your hands might seem minor, but skin absorption is real, and inhalation stings. Keeping containers closed tightly every single time cuts risk and saves money.
Accidents still happen—spill kits nearby save the day. I once watched a coworker handle a spill with nothing but paper towels. We learned better. Prepared spill kits, equipped with absorbents and neutralizers, turn panic into procedure. Washing hands, even after glove use, became a ritual for me. It’s not paranoia; it’s smart.
Disposal trips people up. I’d never dump leftovers down the drain. Lab waste containers, clearly marked for halogenated organics, make it out with the next hazardous waste pickup. I double-check disposal records—keeping these updated helps catch mistakes before authorities do. In the rare cases of fires, I have a Class D extinguisher ready since regular ones just spread chemicals around.
Newcomers often underestimate the risks. I’ve pushed for yearly training, practical sessions, and open conversations about close calls. No silent culture—everyone shares lessons learned. Training doesn’t stop at onboarding; reminders and drills keep procedures fresh. Signs on cupboards and tools in their place show respect for safety, not just for the inspectors, but for every colleague who counts on me not to cut corners.
In chemical spaces, trust builds through diligence. Handling 2,5-Dibromothiophene isn’t about paranoia, but about confidence that grows from doing things right, every day. Those habits protect me, my coworkers, and the work we all want to see finished safely.
Anyone in the chemical trade or research field knows the frustration that comes with a substance that’s only offered in one-size-fits-all packaging. 2,5-Dibromothiophene is not immune to this issue. Some labs might need just a few grams for a round of experiments, while others are scaling up syntheses and wish for containers sized in hundreds of grams or even kilograms. This makes the question about packaging options more than a detail. It can affect budgets, workflow, storage logistics, and even safety.
It’s easy to feel boxed in if a chemical only gets supplied in industrial barrels or tiny sample vials. Smaller labs, particularly in universities or small companies, aren’t about to order a five-kilo drum just to carry out a half-dozen reactions. That’s like buying a barrel of soy sauce for a single stir-fry. Labs with tighter budgets often juggle between sharing reagents and splitting costs, so flexible packaging options let them buy just what they need. In research, unused inventory isn’t just wasted money—it’s a potential risk if the chemicals are hazardous or have a limited shelf life.
Step into the catalog of a reputable chemical supplier and you’ll see that 2,5-Dibromothiophene generally comes in a range of packaging sizes. Suppliers commonly list containers as small as 1 gram, with options going up to 25 or 100 grams. Bulk orders can even be custom-packed for industrial customers. These options acknowledge different uses: small-scale researchers, process development teams, and pilot plants all have different needs.
I remember the hassle of trying to split a 100-gram bottle of a different compound among several groups in a university chemistry building. Oddly enough, the smaller the packaging, the more expensive it becomes per gram. This isn’t just a matter of marketing. Packaging, handling, and paperwork all eat into margins. That cost doesn’t seem significant until you’re the one tasked with managing a department budget or choosing between one more experiment and keeping the lights on.
The convenience of various sizes comes at a price. Small bottles offer less waste and easier handling, but cost more per unit. Bigger packages save cash but can be impractical without the right storage or enough demand to use them up before the chemical degrades. It’s not always about price per gram; sometimes it’s about what makes the most sense for the safety and workload in the lab.
Good suppliers don’t just push out a fixed set of options. They’ll usually list standard sizes but are willing to accommodate special requests for large-volume orders or special packaging—sometimes at an extra charge, sometimes just to keep a client happy. That kind of flexibility promises less waste and more responsive support to scientific work. I’ve found that a quick call or email can sometimes unlock custom packaging or bulk pricing not even visible on a website.
Clear options and open lines to suppliers do more than save money. They make it possible to plan experiments, respond to new developments, and keep a workspace safe. As research shifts and new demands emerge in materials science or pharmaceuticals, these practical details can shape success. Thoughtful supply chains aren’t glamorous, but they are essential—and in the end, they let chemists focus on discovery instead of counting every ounce.
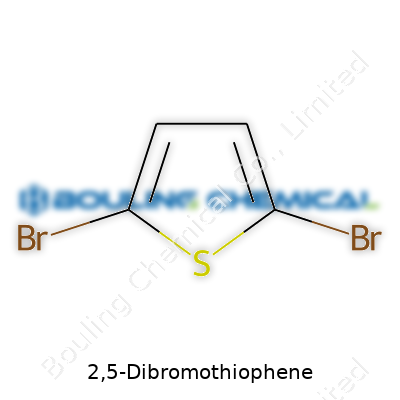
| Names | |
| Preferred IUPAC name | 2,5-dibromo-1-benzothiophene |
| Other names |
2,5-Dibromo-thiophene Thiophene, 2,5-dibromo- 2,5-Dibromothiofene |
| Pronunciation | /ˌtuː.faɪv-daɪˈbroʊ.moʊˌθaɪ.oʊˈfiːn/ |
| Identifiers | |
| CAS Number | 3141-27-3 |
| Beilstein Reference | 120928 |
| ChEBI | CHEBI:71693 |
| ChEMBL | CHEMBL131789 |
| ChemSpider | 108005 |
| DrugBank | DB08407 |
| ECHA InfoCard | 19-2-50561 |
| EC Number | 216-903-5 |
| Gmelin Reference | 394617 |
| KEGG | C18730 |
| MeSH | D004047 |
| PubChem CID | 11330235 |
| RTECS number | WS5850000 |
| UNII | H6L67BT6W7 |
| UN number | UN2810 |
| Properties | |
| Chemical formula | C4H2Br2S |
| Molar mass | 271.92 g/mol |
| Appearance | Colorless to yellow liquid |
| Odor | Aromatic |
| Density | 1.989 g/cm³ |
| Solubility in water | insoluble |
| log P | 2.9 |
| Vapor pressure | 0.0236 mmHg (25°C) |
| Acidity (pKa) | -2.17 |
| Basicity (pKb) | Basicity (pKb) : 6.68 |
| Magnetic susceptibility (χ) | -73.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.640 |
| Viscosity | 2.185 cP (25°C) |
| Dipole moment | 1.90 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 362.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -17.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2717 kJ·mol⁻¹ |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and eye irritation, may cause respiratory irritation, harmful to aquatic life. |
| GHS labelling | GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302 + H315 + H319 + H335 |
| Precautionary statements | P261, P280, P302+P352, P305+P351+P338, P337+P313, P362+P364 |
| NFPA 704 (fire diamond) | 2-2-0 |
| Flash point | Flash point: 88°C |
| Lethal dose or concentration | LD₅₀ (oral, rat): 2530 mg/kg |
| NIOSH | SN8925000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 2-8°C |
| Related compounds | |
| Related compounds |
2,5-Dichlorothiophene 2,5-Diiodothiophene Thiophene 2-Bromothiophene 2,5-Dibromo-3-methylthiophene |