This compound, 2-(4-Methylthiazol-5-yl)ethanol, didn't just show up overnight. Its roots go back to advances in heterocyclic chemistry in the late 1900s, when organic chemists set their sights on sulfur-nitrogen rings for new tools in pharmaceuticals and flavors. Folks realized thiazole rings could open doors, particularly by tweaking the side chains. Moments like the surge in aroma science after the discovery of new volatile components in foods pushed research closer to compounds like this one. Laboratories in both Europe and North America worked on synthesis methods—some for fragrance R&D, some for biochemistry. As chromatography matured, the ability to accurately isolate and characterize small volumes of this ethanol derivative improved, paving the way for safer, larger-batch operations.
Students of chemistry might get introduced to 2-(4-Methylthiazol-5-yl)ethanol through its characteristic earthy, toasted odor, but this is just part of the story. Its structure—thiazole ring bonded to ethanol—offers a blend of reactivity and aromatic flavor that finds use in food science, fragrance formulation, and even medical chemistry. Production scales have moved up from milligram research batches to multi-kilogram runs, with quality control built in thanks to modern chromatographic analysis and digital record-keeping. Product grades vary widely: Some batches suit strict food additive specifications, while others focus on research or industrial applications with relaxed impurity thresholds.
2-(4-Methylthiazol-5-yl)ethanol typically appears as a pale yellow liquid at room temperature, boasting a melting point well below zero Celsius and a boiling point edging above 200°C. Its water solubility lands in the moderate range after heating, due to the ethanol tail, but the thiazole ring precludes high water compatibility compared to straight-chain alcohols. A strong, roasted aroma sets this substance apart from other thiazole derivatives. The hydrogen atoms on the ethanol group present mild reactivity under oxidizing or dehydrating conditions, giving way to possible ester or aldehyde forms, if nudged by the right catalyst. Its refractive index falls in line with comparable aromatic alcohols—something a spectroscopist keeps in mind during purity checks.
Producers catalog this compound by its precise molecular weight, CAS registry number, and purity percentage, often aiming above 98% for applications where taste or health matters. Labels on industrial drums always mention hazard codes tied to irritation or flammability, along with storage conditions—usually a cool, ventilated space. Lot numbers, batch synthesis records, and certificates of analysis ride along with each shipment—no reputable producer skips these, since tracing any problematic batch back to its origin protects everyone. Beyond legal compliance, the big buyers demand transparency on residual solvents and side products, especially if any batch gets close to edible goods.
Synthesis of 2-(4-Methylthiazol-5-yl)ethanol typically starts with commercially available 4-methylthiazole. Chemists add a bromoethanol or haloethanol under controlled basic conditions to drive nucleophilic substitution, attaching the ethanol moiety to the thiazole core. For best results, reaction temperatures stay cool to limit side product formation—overheating intensifies unwanted coupling or ring opening. After reaction, extra solvent washes and column chromatography strip out excess reagents and colored impurities. Final distillation sharpens the purity, and headspace analysis ensures undesirable byproducts don’t sneak onto a sensitive flavorist’s bench. Some labs swap in “green” solvents or solid-supported reagents to reduce waste and improve the safety margins—they don’t want anybody breathing volatile organics that linger long after a shift ends.
Once in hand, the 2-(4-Methylthiazol-5-yl)ethanol molecule offers solid potential for further modification. The ethanol group can undergo esterification with organic acids, producing new esters with intriguing volatility and aroma patterns. Under mild oxidation, this side chain morphs into an aldehyde, possibly altering biological activity or making the molecule more reactive toward nucleophiles. Chemists sometimes substitute or expand the thiazole core to create analogs tailored to specific receptor targets, gradually refining structural activity relationships for pharmaceutical leads. Crosslinking the ethanol moiety with polymers gives rise to flavor-entrapped materials for controlled-release applications in packaging or flavor capsules. Reacting with halogenating agents (with proper ventilation and PPE, always) pushes the molecule toward new classes of derivatives for downstream R&D.
Besides its full IUPAC name, this compound is known in catalogs as 2-(4-Methyl-1,3-thiazol-5-yl)ethanol, or simply as methylthiazole ethanol. In ingredient lists, especially in flavor and fragrance circles, it may turn up as Fema 3254 or under manufacturer-assigned SKUs that carry no hint of its chemistry. In research databases, its CAS number—often 56539-66-3—serves as a sure signpost. Label confusion breeds mistakes, so standardized identifiers like these matter, especially for anyone juggling multiple suppliers or chasing down safety data before a trial run.
Handling this thiazole alcohol commands attention. MSDS sheets warn that vapors may irritate eyes and mucous membranes, while ingestion or prolonged exposure raises concerns about systemic effects on liver or kidney. Labs mandate gloves, goggles, and fume hoods—even if the liquid’s low volatility tricks folks into relaxing their guard. Spills ask for absorbents that neutralize organics; water doesn’t cut it. Good training extends to storage routines: no stacking near strong oxidizers or acids, away from hot surfaces, and drums need regular inspections for leaks. Disposal, of course, follows all regional hazardous waste protocols—this is no time to cheap out, as fine violations stack up fast. Companies in food and fragrance spaces submit not just to local chemical safety boards but also international standards, from REACH to GRAS panels, each demanding a full accounting of toxicology and worker protection.
Probably the best-known use of 2-(4-Methylthiazol-5-yl)ethanol lands in flavor and fragrance formulation, where it delivers roasted, nutty notes to foods and beverages. Instant soups, snack seasonings, and even some chewing tobaccos pull for this compound. Its use stretches into pharmaceutical research, especially since the thiazole ring balances lipophilicity with metabolic stability, inviting medicinal chemists to graft it onto drug leads for antifungal, antibacterial, or CNS-targeted studies. Materials scientists have begun eyeing its reactivity for new polymer additives—quick to point out that even subtle changes in a side-chain alcohol can influence solubility and flexibility. SDS info always requires buyers to declare the exact end-use: a minor note in one bottle turns into a regulatory event if it crosses into any ingestible product.
Ongoing academic and industrial studies dig into both synthesis improvements and new uses for 2-(4-Methylthiazol-5-yl)ethanol. High-throughput screening in pharmaceutical labs often features this building block, thanks to the rich literature connecting thiazole cores to bioactivity. Synthetic chemists explore greener, catalytic routes under solvent-minimized conditions—every production round saved adds up across a big plant. Analytical teams lean on NMR, GC-MS, and HPLC not just for purity, but to fingerprint trace contaminants or structural isomers. Flavor scientists test blends in model food and beverage systems, mapping synergistic and masking effects. Some teams even load the compound onto polymer supports, tracking controlled-release dynamics over weeks—hoping to extend the shelf life of packaged goods or develop next-generation aroma encapsulants.
Animal studies, cell assays, and predictive modeling all chase down the possible risks of exposure. Early toxicology flagged minor hepatic stress at concentrations far above typical food use, prompting follow-up studies on chronic low-dose effects. Engineered cell cultures highlight potential for oxidative stress in liver models, drawing research interest away from bulk industrial application toward carefully dosed, highly-regulated settings. Inhalation risk remains a practical concern in workplace air quality, driving installation of real-time monitoring in flavor plants. Ecotoxicological surveys check for breakdown products in wastewater, because any substance that slips past treatment facilities climbs the regulatory hit list. The drive for safer substitutes or methods for purifying this compound without introducing new side-products keeps medical and environmental researchers busy.
Looking ahead, demand patterns for 2-(4-Methylthiazol-5-yl)ethanol will twist with food trends, medical breakthroughs, and tightening safety law. Flavors that target alt-protein markets—vegan sausages, shelf-stable snacks—may lean ever more on robust roasted notes provided by this compound. Regulatory scrutiny will only sharpen, as consumer pushback against artificial additives gathers steam. On the synthesis front, new routes that bypass hazardous reagents or high-waste processes hold promise; if catalysts based on earth-abundant metals can drop the energy bill or shrink solvent needs, both bottom lines and emissions profiles improve. The drug discovery field bets that subtle tweaks to the thiazole-and-ethanol backbone will yield new classes of bioactive agents, broadening the impact beyond the kitchen or fragrance counter. Industry and academia alike find themselves in a balancing act, stretching known benefits without introducing new risks or burdens—the story of modern chemistry in a nutshell.
Ask any professional in the flavor and fragrance industry to list the chemicals behind those tempting tastes and scents, and you’ll hear about 2-(4-Methylthiazol-5-yl)ethanol. This compound belongs to a class of thiazoles, molecules that deliver a distinctive aroma found in some of the world’s favorite foods. Walk into a bakery or a cheese-making facility, and you may already be catching traces of it without knowing.
Many thiazole-based compounds prove essential for creating flavors that mimic cooked, roasted, or nutty foods. 2-(4-Methylthiazol-5-yl)ethanol brings a savory note—something like the warm, meaty profile you’d smell in grilled chicken or roasted nuts. Food scientists figured out long ago that chemical building blocks could take flavor creation to a new level. For example, researchers at Firmenich and Givaudan, two industry giants, rely on compounds like this one to design both authentic-tasting and innovative flavor profiles.
What makes this particular thiazole valuable isn’t just its aroma but also its stability under heat. A lot of other flavor molecules break down during cooking, but this one holds its own, really supporting taste consistency from factory to dinner table. My background in food science taught me that stability during processing is crucial. A compound that stands up to baking or boiling simplifies the work for product developers.
2-(4-Methylthiazol-5-yl)ethanol appears in the world of fragrances, too. Imagine trying to re-create the comforting scent of fresh bread or roasted coffee. Perfumers sometimes reach for thiazole derivatives as they bottle familiar smells. The compound’s sensory power means it’s effective even at low concentrations, keeping production costs down without sacrificing quality.
Chemists occasionally use thiazole derivatives in synthesis, serving as intermediates for pharmaceuticals and specialty chemicals. Though that role remains a little niche compared to its food and fragrance jobs, it’s not just a one-trick pony. As research pushes deeper into flavor perception and synthetic biology, new applications may pop up, especially as companies seek sustainable flavor sources beyond traditional agriculture.
No flavor chemical reaches your kitchen shelf without scrutiny. 2-(4-Methylthiazol-5-yl)ethanol goes through safety checks by agencies like the Flavor and Extract Manufacturers Association (FEMA) and the European Food Safety Authority (EFSA). They look at toxicity data, dietary exposure levels, and real-world use before giving it the green light. Responsible sourcing and rigorous testing protect consumers, and as someone who’s spent time in regulatory compliance, I know those hoops aren’t just paperwork—they build trust.
Sustainability is another angle to consider. Consumers expect transparency, ethical sourcing, and responsible chemistry. As more companies adopt green chemistry principles, the production of flavor chemicals grows cleaner, with more renewable materials entering the mix. Synthetic routes compete with fermentation-based approaches, and both sides look for a lighter footprint.
Demand for authentic flavors continues to climb, especially with the rise in plant-based and reduced-meat diets. 2-(4-Methylthiazol-5-yl)ethanol and its cousins stand at the intersection of tradition and technology. By supporting robust flavor profiles, they help bridge gaps in taste and satisfaction, enabling food manufacturers to deliver on both nutrition and sensory pleasure.
Open conversation about what’s in our food, combined with tough safety standards and new green chemistry tools, lets science and industry meet expectations without sacrificing joy at the table. As flavor creators and researchers push forward, compounds like 2-(4-Methylthiazol-5-yl)ethanol become more than backroom chemicals—they turn into quietly reliable partners in the foods and smells we love.
2-(4-Methylthiazol-5-yl)ethanol often pops up in discussions among chemists and lab workers who focus on specialty chemicals. I have worked in a research lab where small molecules like this play a key role in synthesizing aromas and specialized compounds. It's clear from experience that not every chemical with a complex name is dangerous, but ignoring the proper protocols can turn minor carelessness into a serious mistake.
Looking at the material safety data, 2-(4-Methylthiazol-5-yl)ethanol tends to rank as a substance requiring careful handling, even if outright hazards seem limited compared to industrial solvents or corrosives. Skin and eye irritation consistently top the list of possible health risks. Sometimes, just a minor exposure leads to itching or redness. In the lab, a whiff during weighing or transfer can create a noticeable itch in the throat or watery eyes. Getting a little on your gloves and then absent-mindedly touching your face makes the risk feel much less theoretical.
As with many organic chemicals, harmful effects often remain subtle at first. There’s usually not much research on long-term effects for lesser-known compounds like this. Without chronic exposure data, it makes sense to take extra care, especially when handling powders, liquids, or reaction residues. From personal experience, most people only make safety a priority after seeing someone get a rash, chemical burn, or asthma flare-up.
In a busy lab, cutting corners can seem tempting— a quick pour and back to your bench, no gloves, or leaving lids open for just a minute. I learned early that these shortcuts tend to backfire. Gloves, goggles, and sometimes a mask quickly become routine, not just because of obvious dangers but because keeping chemicals off your skin and out of your lungs means fewer sick days and less stress.
Using a fume hood offers another dependable way to keep vapors away. Even when something smells faint in the bottle, it may still carry compounds that won’t do your airway any favors. Spills and splashes don’t send advance notice. Cleaning up those messes without the right gear leads to trouble, so keeping absorbent pads, gloves, and eyewash stations close makes sense.
Anyone handing out containers of 2-(4-Methylthiazol-5-yl)ethanol in a workplace needs to provide more than printed labels and instructions buried in a binder. Training should walk workers through each task— showing how to open bottles, measure, and transfer material without making a mess or breathing fumes. Clear signage and regular reminders about what to do in an emergency help prevent hesitation if something spills or splashes.
Most important, there’s value in treating every chemical with respect, even if its hazard profile looks tame at first glance. Asking questions about storage, spill cleanup, and personal protection brings safety into everyday decision-making. Over time, these habits protect everyone, not just the person handling the bottle, but colleagues who share the workspace and the people who clean up after.
In practice, the safety of 2-(4-Methylthiazol-5-yl)ethanol depends on consistent habits and a commitment to looking out for each other. The best labs and factories I’ve seen treat even routine chemicals with caution, and those habits pay off in fewer accidents and healthier teams.
2-(4-Methylthiazol-5-yl)ethanol looks like a mouthful, but breaking down its chemical structure reveals something interesting. The core here is a thiazole ring, which houses both nitrogen and sulfur atoms, matched with a methyl group at the fourth carbon. Tucked onto the fifth carbon, an ethyl side chain ends in a hydroxyl group—that’s the ethanol bit. Pieces fit together to make the full molecule: C6H9NOS. That’s six carbons, nine hydrogens, one nitrogen, one oxygen, and one sulfur.
The reach of a simple molecule can stretch far. I’ve found that molecules like 2-(4-Methylthiazol-5-yl)ethanol pop up in areas as different as flavor chemistry, pharmaceuticals, and sometimes as intermediates in organic synthesis labs. The presence of thiazole rings shines in biology—they show up in vitamin B1 (thiamine), which keeps nerves and the heart running well. Swap in a methyl group or add an ethanol tail, and suddenly, chemists shape how a molecule smells, tastes, or even how it behaves inside the body.
This molecule can look easy to draw, but chemistry makes you respect even “simple” structures. It’s one thing to know the formula—C6H9NOS. Making it pure, making it safe, and documenting every step needs solid protocols. Ethanol groups add flexibility and some water solubility. That’s important in both flavor compounds and drug design. The methyl group changes electron density, which can change how reactive the thiazole ring feels during chemical reactions. Every change, even small, can improve a molecule’s use or reduce a risk—for the chemist and the end-user.
Years ago, working with organic compounds, unexpected byproducts cropped up from impurities sneaking in during the synthesis of related thiazole compounds. Raw data and constant checks became my morning ritual. The lesson stayed with me: every element and side chain matters, not just for regulatory approval but for future users. It’s not enough to chase a bare formula—each atom makes life easier or harder somewhere down the line. That’s the story behind a formula like C6H9NOS.
With nitrogen, sulfur, and an alcohol end, this molecule calls for standard chemical safety—proper gloves, ventilation, attention to storage conditions. Thiazole-based compounds sometimes trigger allergic responses or irritation. Strict labeling and thorough documentation make a difference. Reliable sources provide detailed Material Safety Data Sheets. Those sheets back every move in the workplace, protecting workers and ensuring anyone downstream sees what’s actually inside each flask or bottle.
There’s room for less waste and fewer hazards in the lab and manufacturing environment. I’ve seen greener chemistry methods cut down on toxic solvents when building molecules like 2-(4-Methylthiazol-5-yl)ethanol. Continuous learning—sharing practical, evidence-backed steps—raises the bar. Teamwork between chemists, safety managers, and regulators tightens up processes, trims risks, and improves outcomes for everyone. The formula C6H9NOS isn’t just a string of letters and numbers—it’s a doorway to better practices and smarter choices in chemical science.
Step into any chemistry lab, and you’ll see an alphabet soup of weird names and bottles tucked away on every shelf. 2-(4-Methylthiazol-5-yl)ethanol isn’t something you pick up in the grocery aisle. This compound, known for its role as a flavor ingredient and an intermediate in chemical synthesis, deserves careful storage to stay reliable and safe.
Tucking away a lab chemical isn’t just about making room on the shelf. A compound like 2-(4-Methylthiazol-5-yl)ethanol can turn risky if someone leaves the lid loose or lets it sit out where sunlight pours in. From experience, letting solvents or similar chemicals sit near a window or radiator usually ends up with an off smell or, worse, a risky situation for everyone nearby.
Good science leans on facts. Keeping this compound at a steady, cool temperature keeps it from breaking down or releasing vapors. The material safety data sheet (MSDS) for 2-(4-Methylthiazol-5-yl)ethanol suggests a storage spot below 25°C, away from direct sunlight. Humidity wrecks more than old books—the same goes for sensitive chemicals. Any water sneaking in can spoil the material or make it react in ways you didn’t plan.
People who work with chemicals know better than to shove bottles wherever there’s space. A flammable cabinet or a dedicated chemical refrigerator tends to be the best bet. Labels matter, not just for looking proper, but for knowing what you’re reaching for. I once watched a research student mix up containers with nearly identical labels—nothing serious happened, but the lesson stuck.
Containers do a lot of the heavy lifting here. Glass vials with tight-fitting caps stand out as a clear winner over old plastic containers, especially since some plastics just can’t handle organic compounds and start to dissolve over time. Making sure the lid is screwed on tight keeps the chemical fresh and stops any unwanted fumes from escaping. Forgetting this simple step can leave a sour, strong smell hanging around the lab, or even ruin other experiments stored nearby.
Storage isn’t just a technical chore; it speaks to care for the environment as well. Spilled or leaking chemicals, even in small amounts, add up. Proper storage cuts back on waste and keeps the air, water, and staff a lot safer. Some compounds, especially thiazole derivatives, can attract extra scrutiny due to their reactivity or possible breakdown products. By following secure storage procedures, everyone from lab techs to researchers can help avoid unnecessary exposure and make sure the chemical actually does its intended job.
Training takes the top spot. A well-informed team rarely lets a chemical sit in the wrong spot. Posting clear instructions and checking up on storage conditions turns good intentions into good habits. A quick daily glance, wiping up spills right away, and double-checking that every bottle lands in the right bin beats even the best written policy.
Storing 2-(4-Methylthiazol-5-yl)ethanol boils down to more than following a sheet of instructions. It’s about experience, common sense, and respect—for health, for data, and for the work itself. Keeping this compound in a cool, dry, shaded, and clearly labeled place, inside the right container, covers most of what really matters. From there, a lab earns trust, builds good science, and keeps everyone safe.
Looking for 2-(4-Methylthiazol-5-yl)ethanol offers a glimpse into the world of specialty chemicals. This isn’t something you find at the corner pharmacy. Most searches for this compound lead straight into the world of laboratory suppliers or chemical wholesalers, not consumer retailers or supermarkets. That’s a telling sign. People most often buy it for research purposes, food flavoring development, or pharmaceutical applications.
Many professional labs choose established chemical suppliers for good reason. Reputable sources ensure purity, reliable documentation, and compliance with safety standards. Cheaper, less regulated sources exist, but folks chasing a few dollars in savings sometimes end up with contaminants or incorrect compounds. In my earlier research work, I learned quickly that saving money up front could cost time and safety later. A product data sheet alone doesn’t prove quality. Certificates of analysis, safety data, and supplier accreditation make a real difference.
Buying chemicals without clear documentation or from unknown online resellers risks more than wasted money. Transporting and storing substances such as 2-(4-Methylthiazol-5-yl)ethanol calls for good labeling and clear hazard communication. During my time in academic research, regulatory inspectors always homed in on proper labeling. Nobody wanted to untangle a mystery spill after hours.
Online review sites and specialty chemistry forums tell some stories. One friend of mine shared how a cheap bulk purchase from an overseas site left their lab with months of headaches: missing data, untraceable source, and delays while third-party testing confirmed purity. Turns out, working with established suppliers like Sigma-Aldrich or Fisher Scientific has value—reliable delivery, full documentation, safety support. For research, that peace of mind helps folks get actual results instead of troubleshooting supply chain issues.
Buying this kind of compound isn’t always as simple as filling a shopping cart. In most countries, buyers need to show their credentials—a research license, business registration, or proof of academic affiliation. During my time ordering solvents, our purchasing department always asked for end-use info. Responsible suppliers require it for a reason: Legal controls over specialty chemicals keep counterfeit medicines and unsafe additives out of the market. Genuine sellers value traceability, which makes tracking and recalls possible if something goes wrong.
Online auctions and gray-market sales tempt people seeking speed or lower price, but the risks ripple outward. Widespread stories recount customs delays or even seizures at borders because the right import license wasn’t secured ahead of time. No one wants that hassle.
Rather than hunting for the cheapest website, researchers and developers build relationships with trusted vendors. Calling or emailing a supplier directly often leads to better pricing, volume deals, and insights about new regulations. Getting on the phone beats clicking ‘add to cart’ every time. Reliable sources not only sell a product, but guide buyers through safe shipping, compliant paperwork, and shelf life concerns.
2-(4-Methylthiazol-5-yl)ethanol, like many specialty chemicals, rewards those who put effort into sourcing. Good suppliers stand behind their product and stand with their customers if problems crop up. That makes all the difference for anyone who values their results, their time, and their reputation.
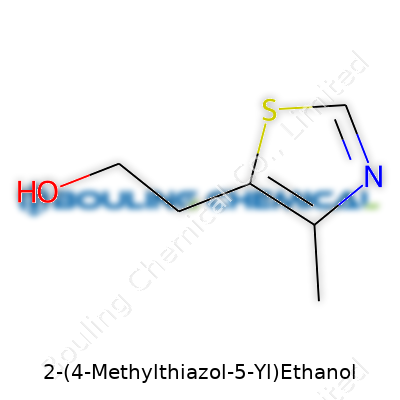
| Names | |
| Preferred IUPAC name | 2-[4-Methyl-1,3-thiazol-5-yl]ethan-1-ol |
| Other names |
2-(4-Methyl-1,3-thiazol-5-yl)ethanol 4-Methyl-5-(2-hydroxyethyl)thiazole 4-Methylthiazol-5-ylethanol |
| Pronunciation | /tuː fɔːr ˈmɛθɪl θaɪˈæzɒl faɪv aɪl ˈiːθənɒl/ |
| Identifiers | |
| CAS Number | [19129-24-1] |
| Beilstein Reference | 2111531 |
| ChEBI | CHEBI:84178 |
| ChEMBL | CHEMBL3249077 |
| ChemSpider | 21415908 |
| DrugBank | DB04156 |
| ECHA InfoCard | 03e871c2-bc60-4831-a5ad-c5fe06f1c1b6 |
| EC Number | 229-555-4 |
| Gmelin Reference | Gm.24.604 |
| KEGG | C06427 |
| MeSH | D000068286 |
| PubChem CID | 123601 |
| RTECS number | KH8575000 |
| UNII | YN8TU787A9 |
| UN number | Not classified |
| Properties | |
| Chemical formula | C6H9NOS |
| Molar mass | 143.20 g/mol |
| Appearance | Light yellow to yellow liquid |
| Odor | wheat, nutty, popcorn |
| Density | 1.167 g/cm³ |
| Solubility in water | soluble |
| log P | 0.17 |
| Vapor pressure | 0.0475 mmHg at 25°C |
| Acidity (pKa) | 14.4 |
| Basicity (pKb) | 14.16 |
| Magnetic susceptibility (χ) | -47.41 × 10^-6 cm³/mol |
| Refractive index (nD) | 1.541 |
| Viscosity | 30 mPa·s (lit.) |
| Dipole moment | 1.98 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 309.2 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -4868.7 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | 99.9 °C |
| Autoignition temperature | 404°C |
| Lethal dose or concentration | LD50 Oral Rat 1,560 mg/kg |
| LD50 (median dose) | LD50 (median dose) = 860 mg/kg (rat, oral) |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10g |
| Related compounds | |
| Related compounds |
2-(4-Methylthiazol-5-yl)ethylamine 2-(4-Methylthiazol-5-yl)acetic acid 2-(4-Methylthiazol-5-yl)acetaldehyde 4-Methylthiazole 2-(Thiazol-5-yl)ethanol |