Long before high-precision analytical tools, chemists relied on trial, error, and quite a bit of intuition to craft more complex aromatic compounds. Interest in heterocyclic chemistry took off as scientists hunted for new materials with adjustable electronic properties. Thiophenes, discovered in the mid-19th century as coal tar byproducts, caught many eyes for their sulfur atom, which introduced both challenges and possibilities to organic synthesis. The arrival of 4-fluoro substituent on thiophene’s aromatic ring stemmed from a wave of interest that blended fluorine’s unique characteristics with sulfur-rich rings. The motivations linked to pharmaceuticals, organic electronics, and even agricultural research quickly made fluorophenyl thiophenes a recurring target in labs. Over time, improvements in fluorination techniques and cross-coupling reactions put 2-(4-Fluorophenyl)thiophene in the toolkits of researchers worldwide.
2-(4-Fluorophenyl)thiophene features a thiophene ring fused with a fluorinated phenyl group at the 2-position. This structure isn’t just academic; chemists favor it because the molecule’s blend of electron-withdrawing fluorine and the sulfur in thiophene makes for an attractively reactive package. The compound finds itself in development for several applications, including organic semiconductors, analytical standards, and as a building block for pharmacologically active molecules. In recent years, catalogues from chemical suppliers list it with varying degrees of purity and tailored for high-performance applications, hinting at its growing role in advanced material research.
At room temperature, 2-(4-Fluorophenyl)thiophene often turns up as a pale crystalline solid or sometimes a light brown powder, influenced by storage and handling. It brings together properties of both thiophene and fluorinated aromatics—moderate molecular weight, significant hydrophobicity, and a tendency to resist breakdown under normal atmospheric conditions. Boiling point hovers between 260-285°C, with a melting range slightly below 60°C. Its solubility shines in organic solvents like chloroform and dichloromethane; water doesn't stand a chance with its hydrocarbon skeleton and low polarity. On the chemical front, it stands up well to moderate acids and bases, but halogenation tends to hit the thiophene ring fastest when strong reagents are around.
Chemical suppliers tag this compound with identifiers such as CAS 55762-41-1 and assure buyers of a minimum purity of 97% for research use. Quality control teams rely on HPLC, NMR, and mass spectrometry to verify identity and composition, flagging up spectroscopic signatures unique to the fluorophenyl and thiophene groups. Labels highlight hazards for skin and eye contact, call out the need for gloves, eye protection, and ventilation, and stress keeping the compound sealed away from light and strong oxidizers. Laboratories who handle large batches log batch numbers and expiry dates, maintaining full trackability for downstream applications.
The most direct way to 2-(4-Fluorophenyl)thiophene connects back to Suzuki cross-coupling chemistry. Chemists pair 2-bromothiophene with a 4-fluorophenylboronic acid, using a palladium catalyst and a base, often potassium carbonate, in solvents like toluene or dioxane. Careful temperature control—usually just below 100°C—keeps side reactions at bay. After workup and extraction, purification typically leans on flash chromatography, given the product’s close boiling and melting points relative to very common byproducts. Some labs experiment with direct C–H arylation, cutting out the need for pre-functionalized starting materials, though yields sometimes lag behind. Multi-gram scale production sticks with proven cross-coupling: it scales up without introducing unexpected impurities.
Plenty of labs select 2-(4-Fluorophenyl)thiophene as a starting point for both electrophilic and nucleophilic substitution. Attaching new groups to the 5-position of the thiophene ring usually succeeds with directed lithiation or Stille coupling. The fluorine on the phenyl ring invites further substitutions, swapping for other nucleophilic groups under harsh conditions, even if reactivity drops compared to a straight-up phenyl ring. Chemists eager to tune the material properties—especially for OLEDs or organic solar cells—explore variations by adding alkyl chains or electron-rich substituents, nudging optoelectronic performance bit by bit. The compound stands up to methyl and ethylations, and esterification using carboxylic acids on available positions rarely presents surprises.
Catalogues and academic journals often alternate between “2-(4-Fluorophenyl)thiophene”, “4-fluorophenylthiophene-2”, or the less wieldy “2-(para-fluorophenyl)thiophene”. Product lists sometimes abbreviate it as “4F-Ph-Thiophene”. Database searches link the structure to its CAS number 55762-41-1, while trade listings attach prefixes for different grades or solvent-specific formulations. Careful check on the IUPAC name, 2-(4-fluorophenyl)thiophene, helps sidestep mix-ups with compounds bearing a meta- or ortho-fluoro tag instead.
Working with 2-(4-Fluorophenyl)thiophene, I make a habit of consulting the safety datasheet before getting anywhere near the lab bench. The compound doesn’t flash readily, but its organic vapor does present a real inhalation hazard if large quantities evaporate or if spills go unchecked. Contact with skin or eyes can cause local irritation; gloves and goggles aren’t optional. Standard fume hoods handle any dust or vapor, but good practice always pairs proper storage—sealed containers, away from acids or bases—and a clear spill response plan. Waste solutions find their way into halogenated organics bins, since local disposal rules don’t go easy on organofluorine residues.
My colleagues and I run into 2-(4-Fluorophenyl)thiophene mostly in the chase for new electronic materials. The mix of electron-rich and electron-poor zones suits design of light-emitting devices, flexible solar cells, and thin-film transistors, where charge mobility matters. Pharma researchers value the compound’s framework as a base for building more complex aromatic drugs, especially those looking for improved metabolic stability or fine-tuned receptor activity—fluorine does wonders for biological half-lives. Outside electronics and medicine, a few teams keep their eyes open for pest control options, banking on the molecule’s hybrid structure to disrupt insect biochemistry.
Exploring new properties of 2-(4-Fluorophenyl)thiophene eats up pages of recent organic and materials science journals. One focus stays on integrating the compound into polymer backbones to lift device efficiency and lifespan in flexible displays. Other labs, especially in pharmaceuticals, use the molecule as a platform for structure-activity relationship testing; swapping out the fluorine or plugging new groups onto the thiophene ring opens up long lists of analogues. Analytical chemists run refinements on detection techniques, making sure contaminants or byproducts from manufacturing don’t slip through in sensitive applications. The research never seems to settle, as new computational models hint at missed reactions or unexplored isomers.
My experience with safety testing leads me to track the literature on aromatic and fluorinated compounds. Most short-term studies peg 2-(4-Fluorophenyl)thiophene as low acute toxicity, with no strong evidence for genotoxic or carcinogenic effects at lab-scale exposures. Chronic data, especially beyond murine and in vitro tests, remains thin. I’ve noticed risk assessments flag the general tendency of organofluorine compounds to resist standard wastewater treatment, raising longer-term persistence and bioaccumulation questions. Local regulatory filings send out warnings about handling with care and minimizing release to sewers or open ground. Until more long-term data comes in, best to treat even this low-toxicity profile compound with respect—good containment, good cleanup, and no loose dusts.
Interest in 2-(4-Fluorophenyl)thiophene keeps growing as materials engineers hunt for the next generation of flexible displays, wearables, and light-based sensors. Synthetic chemists keep tweaking reactions to reduce waste, shorten steps, and cut out expensive metals in the process. Pharma teams stay curious about how subtle tweaks to the aromatic core can offer new routes to block-buster drugs. Environmental scientists look at advanced treatment techniques, trying to figure out how to break down stubborn fluorinated organics before they persist in soil or water. I see a future where bio-based and recyclable routes might replace petroleum-driven syntheses, and where computational design speeds up property optimization before a single test tube hits the bench. The future balance between utility, safety, and environmental responsibility depends on keeping all three in the lab spotlight.
It feels a bit like solving a puzzle, staring at a name like 2-(4-Fluorophenyl)thiophene. The pieces fit together once you recognize what each part signals. “Thiophene” forms the backbone—a five-membered ring with four carbons and one sulfur. At the second carbon, there’s a “4-fluorophenyl” group. That means a benzene ring with a fluorine atom sitting at the fourth spot, counting from the attachment point.
Drawing it out, you get two rings: thiophene and benzene. The bridge between them stands at the thiophene’s number two carbon, reaching over to benzene’s fourth carbon, right where the fluorine sits. The chemical formula ends up as C10H7FS. Those ten carbons, seven hydrogens, one fluorine, and one sulfur each matter, defining how this compound behaves in the wild world of molecules.
A structure like this isn’t just an exercise in organic chemistry. People working in medicinal or materials chemistry run into compounds like 2-(4-fluorophenyl)thiophene all the time. The fluorine alteration seems minor at a glance, but that one atom can flip the properties from sluggish to high-performing. Sometimes the difference between lab success and disappointment boils down to such a tiny switch.
Fluorinated aromatics serve as essential building blocks in drug discovery. Adding a fluorine to aromatic systems can create stronger bonds in metabolic processes, making drugs last longer in the body. In the case of 2-(4-fluorophenyl)thiophene, the combination of thiophene and fluorobenzene rings tends to create molecules that pass through biological membranes more easily. This improves chances for success in the early stages of drug research.
I've worked with a handful of thiophene derivatives in lab settings, mostly focusing on how small changes affect their performance. Even subtle additions, like that fluorine at the fourth position, can send a ripple effect through the entire molecule. Sometimes, a synthesis procedure that works like a charm for ordinary phenylthiophenes trips up when a fluorine gets introduced. The need for new methods, customized conditions, and the occasional troubleshooting session adds hours to what looked like a simple project on paper.
This is the reality of molecular design: progress only comes through patience and a willingness to dig into the nitty-gritty. Strong collaboration between chemists helps overcome these obstacles. Swapping notes about failed reactions, reading the latest journals, and pushing each other to try innovative approaches help everyone move forward.
More accessible databases and advanced software now offer clues about compounds like 2-(4-fluorophenyl)thiophene. Predictive tools save time by flagging synthetic bottlenecks. Another practical solution involves embracing greener chemistry—finding pathways that reduce hazardous reagents, lower waste, and shrink the environmental footprint of making such molecules.
Building a supportive community of chemists, sharing both victories and setbacks in chemical synthesis, lifts the quality of research. It’s not just about knowing the formula or drawing the ring structures. Real progress comes from understanding every corner of these molecules and knowing their value extends well beyond the textbook page.
Labs across the world see a lot of 2-(4-Fluorophenyl)thiophene. My time shadowing organic chemists showed just how valuable this compound gets once research heats up. It’s a solid stepping stone in the quest to build even more complex molecules. Ask someone piecing together pharmaceutical candidates — they turn to thiophene rings like this one to bring new characteristics to their chemical toolkit. By adding a fluorine atom to the phenyl side, you don’t just tweak the look, you change the way the molecule behaves, too. Chemists thrive on this kind of flexibility.
Medicinal chemistry seems hungry for these sorts of building blocks. Structures like 2-(4-Fluorophenyl)thiophene regularly show up in early-stage drug design. Researchers want molecules that stay stable, can pass through cell membranes, or dodge the body’s metabolic clearance systems. Fluorine atoms help with all that. They’re small, sharp, and shield the rest of the molecule in clever ways. From anti-inflammatory agents to CNS drug candidates, structures like this keep finding new uses every year.
Walk into a conference poster session on organic electronics, and you’ll notice students echoing the same names — thiophene derivatives and their friends. Flexible displays, OLEDs, even experimental solar cells: all of them rely on clever carbon chemistry. Here, fluorinated molecules bring extra punch. They influence properties like electron mobility and thermal stability. This isn’t just academic talk — performance jumps out when you tweak the molecule just right. Add a fluorine group and suddenly the material lasts longer, carries charge better, or even glows in a new way.
The world of synthetic dyes thrives on subtle chemical tweaks. A little fluorine can shift a pigment’s brightness, resistance to sunlight, or solubility in ways you only appreciate if you’ve struggled mixing your own inks or paints. I watched a startup screen dozens of molecules searching for the right color-fastness in outdoor textiles. Compounds like 2-(4-Fluorophenyl)thiophene stood out for their strength under real-world use — not just in the test tube.
Every time a new tool enters the workbench, there’s a responsibility baked in. Building with fluorinated aromatics demands careful waste handling and smart regulation. Some of these compounds stick around in the environment. You can’t toss them out like yesterday’s sandwich. Teams working with thiophene derivatives put strict protocols in place — solvent recovery, containment, deep review before scaling up. Dealing with persistent compounds isn’t anyone’s highlight, but it keeps the labs safer for everyone involved.
The story of 2-(4-Fluorophenyl)thiophene reflects a bigger movement in chemistry — coaxing more from every atom, learning from small changes, and taking responsibility for the impact. Moving forward, green chemistry approaches and new catalysts could shape how researchers use these fluorinated rings. Wiser design choices on the lab bench ripple out to better medicines, brighter displays, and safer factories. You appreciate how every molecule, down to a single fluorine swap, pulls a long thread from lab experiment to finished product.
My early days in the lab taught me one universal rule about chemicals—they don’t care if you underestimate them. The name “2-(4-Fluorophenyl)thiophene” might sound like something you’d only see in a grad student's thesis, but it deserves the same respect as any more notorious reagent. Just like bleach or paint thinner, this compound has risks, and brushing those off can add up to trouble.
The right spot for 2-(4-Fluorophenyl)thiophene isn’t an afterthought. I’ve seen folks toss bottles onto the nearest shelf, but one spill can turn a simple day into an emergency. I always opt for a cool, dry, and dark place—think locked chemical cabinet, not next to the coffee supplies. The fewer people have access, the better. Fumes and light love to play tricks with chemicals, so a tightly sealed amber glass bottle usually gets my vote. Direct sunlight tempts reactions nobody wants.
I never store it near oxidizers or acids. Mixing chemicals, even by evaporation or leaky containers, seems innocent enough until you hear the fire alarm. One fact sticks: 2-(4-Fluorophenyl)thiophene can give off harmful gasses if it breaks down or reacts. That’s one more reason to keep containers clean, undamaged, and labeled in plain language.
I learned fast to glove up, even in “just a second” situations. Splashing it on bare skin or getting a whiff of concentrated vapor leads to more than just discomfort—even tiny doses can bring irritation. We use nitrile gloves, not latex, because the wrong glove gives a false sense of safety. Goggles always go on, no matter what. Chemical-resistant aprons actually matter, especially if you’re prone to accidental spills.
Nothing replaces good ventilation. Fume hoods aren’t decoration; they pull away fumes before you breathe them in. If you ever worked with strong-smelling solvents, you know just a little vapor can chase you from the room. The same goes for this compound, especially during transfers or heating. Never pipette by mouth, even if you saw old-timers do it—one mistake, and you’re calling the doctor.
I’ve seen too many people gamble with disposal. Pouring leftovers down the sink felt normal until somebody had to explain the dead fish to the wastewater crew. Chemical waste needs its own properly-labeled container, stored until a trained tech can remove it. In case of a spill, absorbent pads and neutralizers help, but you want that spill kit prepped before trouble hits, not after.
Eye-wash stations and safety showers look useless—until the day you need them. Walk through the process with co-workers, because in the moment, nobody wants to fumble looking for instructions.
It’s easy to get busy, cut corners, and hope things stay safe. From personal experience, short-term laziness brings long-term regret. Following sound rules protects people and keeps projects on track. With chemicals like 2-(4-Fluorophenyl)thiophene, respect isn’t just for the handbook—it’s a way to make sure everyone gets home in one piece. Better habits come from knowing the risks, not ignoring them.
Chemistry enthusiasts and scientists know the difference between reliable results and wasted time often boils down to purity. 2-(4-Fluorophenyl)thiophene—an organic compound featuring both fluorine and sulfur—has purity levels that can swing research outcomes or production quality. In my own lab experience, anything lower than 97% for organics often causes headaches. Reagents bring along unwanted side products, and those can wreck an experiment in ways you usually notice far too late.
Commercially, the most frequent purity rating you’ll see for 2-(4-Fluorophenyl)thiophene sits at 97% or 98%. Chemists usually start with this range for chemical reactions or analytical work, but higher grades, sometimes up to 99%, get used for more specialized work or pharmaceutical routes. Precise purity matters, especially for pharma and electronics, where a percent off translates straight into failed batches or compromised performance.
In cases where purity is critical—think in the last stage before introducing a compound into preclinical testing—companies will offer this chemical with certificates of analysis, showing impurity profiles clear as day. Without it, you’re trusting luck, not chemical science.
If you’re ever the one tasked with ordering chemicals, you know how packaging size can be surprisingly tricky. Buy too much, you watch money evaporate as shelf time leads to slow degradation or useless leftovers. Too little, and you’ll slow your team down with needless reorder cycles. With 2-(4-Fluorophenyl)thiophene, I’ve run across bottles starting as small as 1 gram or 5 grams, climbing up to 25, 50, even 100 grams for academic and industrial applications.
Suppliers mainly opt for amber-glass bottles, aiming to shield from light and air. For small-scale lab use, 1, 5, or 10 grams match most synthetic chemists’ routines. Medium outfits or pilot plants lean towards the 25 or 100 gram scale. I’ve seen bulk inquiries requesting kilos; those always involve extra paperwork around shipping, storage, and legal compliance, especially if you’re across borders or working in regulatory-heavy environments.
It’s not just a matter of availability: packaging reflects cost per gram, storage space, and safety rules. Opening too big a bottle too often means chemical breakdown or unexpected risks. No one wants to gamble with reactivity, accidental spills, or contamination from moisture and air.
Over the years, I’ve watched teams hit setbacks because they underestimated the importance of tight purity specs and well-matched container sizes. The supplier’s job doesn’t end at shipping a bottle—it extends to documenting lot history and helping customers plan out stocks to avoid expired or contaminated product.
Quality control on the client side—especially in places without big budgets—means relying heavily on the accuracy of supplier data. For research groups or startups, it gets tricky because every milligram stretches the budget, but cheaping out on purity ends up far more expensive. Purity guarantees become insurance against wasted cycles and repair work.
So there’s a real case for better cooperation between suppliers and end-users, where labs communicate expected use and shelf life, and companies respond with smarter packaging, batch traceability, and access to higher-purity lots for validated processes. Transparency in documentation and responsible handling—supported by both sides—lets real science happen.
Spending time in a lab teaches you to respect every bottle on the shelf, no matter how innocuous the name sounds. 2-(4-Fluorophenyl)thiophene might not stand out to the untrained eye, but working with anything containing both fluorine and sulphur in the same molecule deserves a bit of extra thought. You can look at chemical databases and see phrases like “irritant” or “handle with care,” but what does this actually mean in practice?
My lab notebook has a section titled “Don’t Skip These Steps,” and this compound would certainly make the list. The fluorine atom, tucked on the aromatic ring, often signals increased mobility through biological membranes. That means the chances for skin or respiratory uptake can inch a little higher than with some other organics. Some folks get cavalier, thinking gloves and goggles are just for the novices, but it only takes one slip to remember why those rules came about in the first place.
Volatility isn’t the biggest concern with 2-(4-Fluorophenyl)thiophene compared to very light solvents, but one can’t call it harmless. A spill can linger on the bench, and the sharp, sometimes almost sweet chemical smell is a clear sign to crack open the fume hood. Fluorinated aromatics have a track record for causing respiratory irritation, and while this isn’t as notorious as some, I still wouldn’t pipette it by mouth or let it splash on skin.
Anyone who has felt the sting of a chemical splash knows personal protective equipment buys valuable time. Even so, the story doesn’t end with gloves. Compounds like this have a habit of sticking to surfaces or even clothing, and sometimes the signs of irritation don’t show up until hours later. Eye protection and a good habit of washing up after handling, not just before lunch, makes a noticeable difference in how often people need to fill out the incident log.
Labs produce more waste than anyone wants to admit, and the disposal section of any protocol always feels a bit too short. For 2-(4-Fluorophenyl)thiophene, you don’t want to pour it down the drain and call it a day. Fluorinated organics tend to hang around in water and soil longer than regular hydrocarbons. Those persistence issues echo into worries about bioaccumulation and damage that goes far beyond one small spill on the lab counter.
No one wants their experiment to turn into a local pollution problem. Proper containment and working with environmental safety officers to handle waste makes the difference between a smooth project and unintended consequences down the road. The costs can add up; I’ve seen research budgets sink when a university learns the hard way about improper solvent disposal.
Getting training isn’t just ticking a box before a project. You pick up shared knowledge — a trick for double-bagging certain bottles, a shortcut to neutralize small spills, a list of firm “don’ts” that everyone who’s handled reactive organics swears by. Adopting clear labeling, not hurrying in the hunt for data, and having working spill kits close at hand turn chemical handling from a gamble into a manageable routine.
Researchers, students, even the maintenance crew—everyone shares the risk and the responsibility. There’s no heroism in skipping gloves or cranking open a bottle outside the hood. I’ve found that the best-run labs keep the group honest, and that culture does more for safety than any checklist ever could.
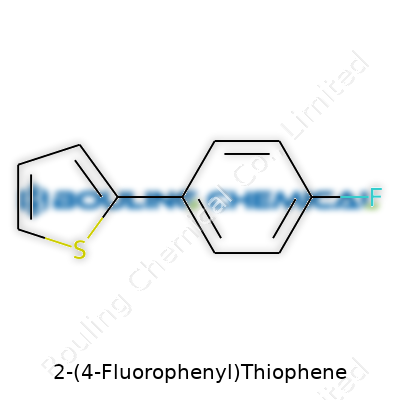
| Names | |
| Preferred IUPAC name | 2-(4-fluorophenyl)thiophene |
| Other names |
4-Fluorophenylthiophene 2-(4-Fluorophenyl)thiophene 2-Thienyl-4-fluorobenzene |
| Pronunciation | /tuː fɔːr fluːrəˈfiːnɪl θaɪəˌfiːn/ |
| Identifiers | |
| CAS Number | [35612-53-6] |
| Beilstein Reference | 1201147 |
| ChEBI | CHEBI:130402 |
| ChEMBL | CHEMBL151162 |
| ChemSpider | 167882 |
| DrugBank | DB08322 |
| ECHA InfoCard | echa.europa.eu/substance-information/-/substanceinfo/100.059.937 |
| EC Number | 828-153-2 |
| Gmelin Reference | 119873 |
| KEGG | C14748 |
| MeSH | C568262 |
| PubChem CID | 69740 |
| RTECS number | XN8570000 |
| UNII | QMW86C44XW |
| UN number | UN3438 |
| CompTox Dashboard (EPA) | DTXSID10901702 |
| Properties | |
| Chemical formula | C10H7FS |
| Molar mass | 180.22 g/mol |
| Appearance | Light yellow to yellow liquid |
| Odor | Odorless |
| Density | 1.238 g/cm³ |
| Solubility in water | Insoluble in water |
| log P | 3.7 |
| Vapor pressure | 0.0165 mmHg at 25°C |
| Acidity (pKa) | 4.46 |
| Basicity (pKb) | PKB = 16.61 |
| Magnetic susceptibility (χ) | χ = -69.37·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.597 |
| Dipole moment | 2.98 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 221.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -5739.7 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P264, P270, P280, P301+P312, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | 101°C |
| NIOSH | NA9260000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 2-(4-Fluorophenyl)Thiophene is not established. |
| REL (Recommended) | 10 mg/m3 |
| Related compounds | |
| Related compounds |
Thiophene 2-Phenylthiophene 4-Fluorophenylboronic acid 4-Fluoroiodobenzene 2-(4-Chlorophenyl)thiophene |