Scientists have studied aromatic amines for well over a century, often in pursuit of new dyes or pharmaceutical breakthroughs. 2,3-Phenazinediamine came out of this wave of curiosity in the laboratories of early organic chemistry, just as researchers learned to build complex ring systems. Early documentation of phenazine derivatives, stretching back to the late 1800s, focused on their vibrant coloring and biological activity. Chemists in postwar decades went much further, learning to manipulate these molecules and chase variations that offered diverse chemical characteristics. Curiosity about the phenazine core led teams to synthesize and characterize derivatives like 2,3-Phenazinediamine, hoping to unlock more robust dyes or antibiotics. What makes this story interesting isn't just the chemical achievement, but the persistence needed—batches would often come out impure and protocols called for endless tweaks before anyone could get a handle on real properties or bioactivity.
You’ll find 2,3-Phenazinediamine turning up in both research and industrial settings, thanks to its two amino groups and that familiar fused ring core recognized across the phenazine class. On hand in the lab, it shows up as a dark solid, catching the attention of anyone working with redox-active dyes or looking to modify natural products. Because its chemical backbone links up neatly with both aromatic and heterocyclic chemistry, researchers can test it as a stepping-stone compound for synthesis, or tweak it to serve as a starting material for more specialized molecules. These connections help drive its importance in development of sensors, new antibiotics, and even sometimes the creation of functional polymers. Whenever a niche needs a stable, easily modifiable diamine, this compound sits high on the list.
Holding a solid form at room temperature, 2,3-Phenazinediamine usually presents in a dark or reddish color. Chemists respect its stability in dry storage, though moisture and air exposure prompt caution because impurities can sneak in and change results. Solubility depends on the choice of solvent—higher in polar aprotic media, but it’s sluggish in non-polar ones. In terms of reactivity, its two amino groups and rigid conjugated core encourage substitution and redox activities. That highlights its dual personality: stable enough for handling, yet reactive enough to anchor both basic studies and modification for practical applications. The melting point speaks to purity, and historical accounts show variability tied to the method and diligence in recrystallization. It may not stink up the bench, but it has a punch if inhaled or mishandled, so common sense and gloves rule the day.
Staring at the label on a 2,3-Phenazinediamine jar, you’ll notice both IUPAC designation and typical specs. Purity levels can run from technical grade (suitable for broad, less sensitive work) to analytical grade, topping 98% for high-end synthesis or precise kinetic studies. The CAS number 92-54-6 gives suppliers and scientists common ground in ordering or research. Guidance runs clear about keeping it in sealed, dry containers, away from oxidizers and sources of ignition. Documentation from suppliers lays out specification sheets, reflecting physical form, melting point, moisture content, and presence of side products, since synthesis routes don’t always shut down side reactions cleanly. Experienced chemists scrutinize these sheets before taking on sensitive syntheses, as trace impurities in diamines can muddy interpretation in downstream chemical or biological studies.
Making 2,3-Phenazinediamine calls for careful reduction or substitution chemistry. The most established route starts either from phenazine or one of its nitro-substituted precursors. Through catalytic hydrogenation or stepwise reduction, chemists knock down nitro groups to amino functions, sometimes using tin(II) chloride, iron filings, or catalytic palladium and hydrogen gas. The success of any batch depends on keeping reaction conditions clean and controlling temperature—runaway synthesis leads to overreduction, ring opening, or caked-up byproducts. Smaller-scale labs often purify product via recrystallization, catching those elusive high-purity crystals. Industrial routes can scale up, but the balancing act between product yield and practical cost remains. Anyone who’s tried to run these reactions knows the value of patience; overzealous reduction or impure starting material makes for frustrating post-reaction workups.
The structure of 2,3-Phenazinediamine, complete with two amino groups on the phenazine ring, sits ready for chemical adventure. N-alkylation draws crowds for custom building functionalized molecules—chemists design dyes, electrolytes, even potential drug leads by attaching all sorts of side chains to either amine. Diazotization and coupling reactions open pathways for further aromatic substitution, while oxidation sets up transformations toward other phenazine-based molecules, some with pronounced utility in the pharmaceutical or electronics fields. Its redox activity, a hallmark of phenazines, positions it well for investigation as both electron donor and acceptor, leading to uses in sensors, battery technology, and catalytic systems. Those same amino groups create handles for further cross-coupling, condensation, or even coordination with metals in catalyst platforms.
Folks in the lab shorthand 2,3-Phenazinediamine to “o-Phenylenediaminophenazine”, reflecting its structure. Other synonyms, including 2,3-diaminophenazine, underscore how systematic names map onto chemical skeletons. Regulatory documentation or older literature sometimes offer alternative spellings or even legacy trade names, sometimes inherited from dye chemistry days or supplied under in-house labels for catalog companies. In any database search, plugging in the CAS number 92-54-6 helps resolve ambiguity between these synonyms, dodging confusion over similar-sounding compounds.
Dealing with 2,3-Phenazinediamine requires respect for both its chemical reactivity and potential health concerns. Laboratory safety protocols treat the compound as potentially harmful—direct skin contact, eye exposure, and inhalation all warrant gloves, goggles, and adequate ventilation. Safety data sheets carry warnings about possible mutagenicity, based on aromatic amine structure, and emphasize careful waste disposal. Spills call for containment and careful cleanup, sparing the drain or trash bin without neutralization. Longer-term storage guidelines ask for dark, cool spaces away from oxidizing agents and open flames, since heating can drive decomposition or lift toxic fumes. In industrial settings, controls extend to workplace monitoring and clear labeling in storage areas, preventing accidental mix-ups.
2,3-Phenazinediamine finds purpose in lab syntheses and specialty industry efforts alike. Researchers like using it as a precursor for designing new dyes and pigments, banking on the colorfastness and stability features baked into the phenazine core. Pharmaceutical scientists draw on its structure when probing antibiotic analogues or testing for biological activity, especially because some phenazine derivatives show strong antimicrobial and anticancer effects. Its redox chemistry gets attention from those working on electrochemical sensors or battery components. Materials scientists value its use in the modification of polymers, introducing conductivity or other functional properties. Each community draws on the molecule’s reliable reactivity, pushing boundaries in color chemistry and health research.
Academic and industrial labs pay sustained attention to 2,3-Phenazinediamine, with research projects spanning synthesis, modification, and application testing. Pharmaceutical groups assess its core as a scaffold for designing antibacterial or antifungal agents. Materials scientists incorporate it into polymers or investigate performance in dye-sensitized solar cells. Analytic chemists work on optimizing detection systems where the unique electronic transitions of phenazine derivatives prove useful for tracking reactions or monitoring environmental changes. The state of research keeps evolving, powered by easy access to the parent diamine and a growing network of literature sharing best synthetic practices, failure points, and safety observations. Steady improvements in purification and structural modification continue yielding new applications, both in academic papers and patent filings.
Questions about the safety of aromatic amines date back decades, since certain compounds in this group turn up as mutagens or carcinogens in both lab studies and real-world exposures. Toxicity screens for 2,3-Phenazinediamine indicate hazards if inhaled, ingested, or absorbed through the skin, and animal studies report effects tied to irritancy and possible organ toxicity. Researchers take warnings about mutagenicity and long-term exposure seriously, especially for chronic use or scale-up scenarios. Regulatory authorities now demand standardized handling, with chemical hygiene plans spelling out routes of exposure and protective barriers. While acute toxicity appears manageable with personal protective equipment, the compound always gets the label of “handle with care.”
2,3-Phenazinediamine reveals no signs of fading from the chemical landscape. With rising interest in clean energy and advanced materials, research targets include its use in redox flow batteries, organic semiconductors, and next-generation dyes. Pharmaceutical research continues, as antibiotic resistance drives scientists back to phenazine frameworks for alternative drug design. Advances in synthetic methodology will matter—better, greener, or more selective routes to this compound could lower costs and expand its reach into developing countries or industries running on tight budgets. Safety improvements, both in production and downstream usage, get attention too, since environmental and health regulations only grow stricter. Collaboration across chemists, engineers, and health professionals will push forward both innovative applications and responsible management.
2,3-Phenazinediamine has a name long enough to make anyone stumble. But it holds a tight spot in many industries, often flying under the radar of most people’s daily lives. In crystal or powder form, this compound carries a deep color, hinting at its punch as a dye. It comes out of the labs as a building block in everything from colorants to specialty electronics.
Most regular folks first run into 2,3-Phenazinediamine in the world of dyes. Textile manufacturers have relied on phenazinediamines for years because they create deep, resistant colors that don’t fade with a few washes. Think of the bright magenta or burgundy that hangs around after dozens of laundries—there’s a good chance phenazine-based dyes played a role. I’ve seen older factory shirts tossed through heavy-duty cleaning and still holding color, much of that thanks to stable compounds like this.
On top of clothing, these dyes stretch out to plastics and inks. Industrial printers use inks containing derivatives of this compound to hit some tough color standards, making sure labels, packaging, and technical prints come out just right. Inkjet inks and even some marker pens lean on stable color chemistry connected to phenazinediamines.
Researchers spotted long ago that phenazine compounds can fight off microbes. Doctors and biochemists turned some versions into powerful antibiotics. Lab results have shown that derivatives related to 2,3-Phenazinediamine punch holes in bacterial defenses, especially when older drugs slip up. Hospitals battle superbugs every day, so new chemical options don’t stay on the shelf for long.
Work in this field never really ends. COVID-19, antibiotic resistance, emerging diseases—all push scientists to test new molecules. Recently I read about trials where phenazine derivatives made a difference against bacteria found in chronic wounds or hospital settings. It’s gritty, hopeful work fueled by the tireless urge to outsmart germs.
The drive for better electronics has led chemists to re-examine many classic compounds. 2,3-Phenazinediamine shows up in specialty batteries and sensor devices due to its ability to carry electrons in predictable patterns. Lithium-ion batteries and fuel cells need materials that won’t break down or cause leaks, so researchers test phenazinediamines under pressure and heat to see where they hold firm.
I’ve met engineers working on better sensors for breathalyzers and chemical detectors who praise the reliability of phenazine rings. These compounds can take a beating and keep giving steady readings, even in harsh environments. They help devices in everything from pollution tracking to medical monitoring.
Working with any strong chemical invites responsibility. 2,3-Phenazinediamine can irritate skin and lungs, so good lab practices matter. Factories push for better safety training and personal protective gear. No shortcut beats careful storage and handling. Regulators keep a close eye on where these compounds end up, especially near water supplies or common waste.
Chemists keep searching for safer alternatives and greener processes, such as creating the compound in closed systems or finding ways to recycle leftover dye that would otherwise wash down drains. These efforts protect both workers and the environment, tying industrial progress to everyday health.
In the lab, folks rarely joke about 2,3-Phenazinediamine. This stuff brings out a cautious side in scientists for good reason. It isn’t like working with water or baking soda. Forgetting basic protection can mean some serious health risks, and I've seen enough cautionary tales to know it pays to stay sharp around chemicals like this.
Get this compound on your skin and you risk irritation, rashes, or worse. Inhalation, even in small amounts, might mean headaches or long-term trouble with your airways. If it touches your eyes or makes its way to your stomach, hospital visits aren’t off the table. I once watched a rushed tech splash an aromatic amine close to his eyes—an hour with the eyewash station is all it took for everyone else to start double-checking goggles before starting work.
Grabbing gloves, lab coats, goggles, and a face mask feels like a routine. Skipping those steps, though, always invites risk. Nitrile gloves act like a barrier and are nearly mandatory. That lab coat doesn’t serve style points, it keeps tiny spills from hitting your torso and arms. Even simple dust masks or respirators cut the odds of inhaling trouble.
Ventilation wins every time. A well-functioning fume hood should not sit idle. Even a minor slip with this compound can fill the air with stuff you don’t want in your lungs, and a decent air draw whisks those dangers right away.
Leaving traces behind isn’t fair to the next person at your bench. So cleaning spills fast and right takes priority. You always want to check the safety data sheet nearby. Most recommend soaking up spills with inert material, then sealing it in a labeled container. Never toss it into regular trash or sinks—special bins and hazardous waste bags have a real, unflashy job: keeping others safe weeks or even months later.
Training sometimes feels like another hoop set up by administration, but the difference shows the first time real trouble strikes. I’ve benefited from crash courses on spill management, and most pros will agree—walking into a lab without knowing how to use an eyewash, or not understanding local evacuation routes, just piles up risk.
Dangerous chemicals ask for respect, not dread. If labs invest in regular refresher training and keep up visible, clear signage, more folks go home healthy. Emergency equipment like eyewash stations and showers need frequent checks. I always look for labels and data sheets: those little notes can save hours of panic and piles of paperwork.
Handling 2,3-Phenazinediamine—just like lots of potent lab chemicals—doesn’t need to be scary or mysterious. By planning ahead, wearing the right gear, caring about your coworkers, and keeping your head in the game, risk stops being a guessing game and just becomes part of the daily routine. That’s how you keep things safe and simple.
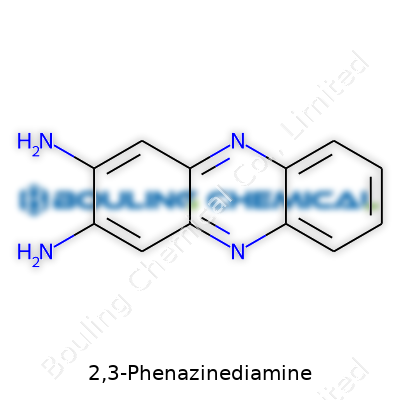
2,3-Phenazinediamine might sound like something that belongs in a thick textbook, but at its core, it's a molecule with a specific set of jobs and risks. To get the basics out of the way, its chemical formula is C12H10N4. This isn’t just a collection of letters and numbers—each element points to a structure that can power real-world applications and also bring up safety questions. The structure, to break it down, involves a phenazine backbone: that's two benzene rings fused together, held tight by two nitrogen atoms. The "2,3-diamine" label means you’ll find amine groups sticking out from the second and third positions on that backbone. These amine groups, NH2, change how the molecule behaves inside a lab or a factory. If you’re drawing it, you’ll notice those extra bits hanging off, influencing reactivity and interaction.
Most folks outside of chemistry circles have barely heard of phenazines, let alone their relatives like 2,3-Phenazinediamine. But behind the technical language sits a connection to dyes, antibiotics, and sometimes even bigger problems—environmental and health effects that follow from how we use and discard chemicals. Years ago, while working in a lab evaluating potential dye molecules, I watched how a small tweak in molecular structure changed everything: color, stability, and the way a compound hung around after use. 2,3-Phenazinediamine has properties that slot into this kind of story. Its amine groups make it a suitable building block for dyes, particularly those with deep purples and reds. At the same time, these groups can lead to side reactions, some helpful, some much less so.
This molecule's reach doesn’t stop at coloring things. In pharmaceutical chemistry, related compounds sometimes turn into antibiotics or cancer drugs. They work by interfering with microbial life or rogue cells but bring safety challenges along for the ride. If you ever helped dispose of old chemistry sets, maybe you’ve seen the headaches of dealing with unknown substances. The same challenge crops up on an industrial scale—waste handling, worker exposure, and spills are real concerns, especially with aromatic amines, which some research ties to carcinogenic risks. No one wants to see a useful tool end up as a health hazard.
Every molecule with power asks for vigilance. Regulations—OSHA guidelines, REACH restrictions in Europe—set basic thresholds for exposure, but in practice, safe handling comes down to habits in the lab and the factory. Installing proper fume hoods, double-checking protective gear, and paying attention to disposal steps all cut risks without much fuss. In past projects, I saw how even a simple checklist caught missed hazards before they caused trouble. It’s easy to focus on what a chemical can do and skip over how it can bite back if ignored.
As science and industry keep hunting for new uses—maybe new dyes, maybe a medicine that makes it through the pipeline—the bones of 2,3-Phenazinediamine and its cousins will keep popping up. The learning curve stays steep because every tweak in the molecule can take its use and risk profile somewhere unexpected. The value lies partly in keeping our eyes open and our rules flexible, so new uses don't lead to new surprises nobody’s ready for.
2,3-Phenazinediamine isn’t a household name, but folks in labs know it carries risks. I’ve seen plenty of cases where neglecting a chemical’s quirks led to ruined supplies or worse, safety scares. This compound tends to oxidize in the open air and loves to stain your gloves and bench tops, so treating it right matters. The dark color and slightly musty smell give away how reactive it can be if the lid’s left loose or the humidity jumps up. The right precautions mean fewer headaches and safer work for everyone in the building.
Leaving 2,3-Phenazinediamine on a shelf with just a twist cap invites all sorts of trouble. I’ve learned the hard way that chemicals like this do not forgive lazy storage. Air, heat, and light will each speed up degradation. Keep it in a tightly sealed glass bottle, ideally amber-colored, to block out stray light. Light triggers changes in phenazine structures, leading to decomposition or unknown byproducts. Saving on fancy containers ends up more costly when you toss out contaminated or decomposed batches. Glass beats plastic every time for holding strong against permeation or chemical attack, and a solid Teflon-lined cap will handle that tendency to stain and leach.
Temperature control means a lot with so many lab chemicals. I keep my bottle stored at cool room temperature, never above 25°C. Refrigerators may work for super-sensitive samples, just never let condensation or moisture sneak into the bottle. Humidity turns fine crystals into sticky clumps that block pipettes and spoil weighing accuracy. A properly dried sample is worth more than twice the amount of powder kept careless in the air. I always add a little silica gel pack in the secondary container—those small steps go a long way. I’ve yet to see a properly sealed, cool-stored batch go bad, even if it takes months to get used up.
Labels don’t just help the next shift or new hires—they keep accidents at bay. The label must clearly say “2,3-Phenazinediamine,” list the hazard codes, and show the date received. Unmarked powder can end up in the wrong reaction, causing mess or even real danger. I’ve caught bottles kicked to the back of shelves with no clear hazard signs; those can cause accidents if someone reaches for them during a busy synthesis. It’s good practice to store this chemical away from acids, oxidizers, or anything it could react with if spilled. In my lab, we dedicate a separate cabinet for aromatic diamines—those mixtures tend to react under the right conditions.
Over time, every lab sees a drop or two hit the floor. Phenazinediamine will stain tile and skin, sometimes for days. Quick action means using plenty of absorbent material, gloves, and a splash of dilute bleach for cleanup. For disposal, don’t pour the remains down the drain. We collect all waste in a labeled, sealed metal drum and arrange pickup by specialized contractors. People forget that incomplete cleanup creates hazards for the next person walking by, and mixing chemical wastes just multiplies problems. Proper disposal prevents environmental contamination and fines from regulators.
Good storage habits with 2,3-Phenazinediamine cut down risk and waste. Every time I store a new batch right, future work goes easier and no one deals with ruined reagents. It comes down to tight seals, cool spaces, clear labels, and keeping incompatible chemicals apart. Anyone working with reactive organics picks up these lessons early—ignoring them means risking health, supplies, and project deadlines.
2,3-Phenazinediamine, a chemical that doesn’t often make headlines, has certain properties that deserve close attention. In chemistry labs, it appears as a dark powder—used in the creation of dyes and sometimes studied for its antimicrobial potential. Its structure puts it in the category of aromatic amines, and if you’ve plodded through safety training, you’ll already know this group can throw up health flags. Even as someone who’s only handled such compounds with gloves on, I recognize the uneasiness that comes with unfamiliar substances like this one.
The effects of 2,3-Phenazinediamine on people aren’t documented as extensively as those from more common chemicals. Still, cousin compounds in the phenazine family have built a rough reputation. Studies have tied them to skin and respiratory irritation. Some aromatic amines have proven to be even more troublesome, with links to mutagenicity and even cancer. These risks aren’t pulled from thin air—history shows workers in dye factories paid the price for unchecked exposure.
Lab tests hint at similar caution for 2,3-Phenazinediamine. If it comes in contact with skin, rashes and inflammation might follow. If fumes waft up during heating or processing, you could end up with upper airway irritation, coughing fits, and headaches. Swallowing it, a thankfully rare accident, could trigger nausea or worse. Based on its chemical kin, one can reasonably expect chronic exposure to pose nastier health risks over time, especially if basic protections get skipped.
Many people never have to pronounce “phenazinediamine,” yet lessons learned with chemicals like this one ripple everywhere. Handing over the safety manual and calling it a day simply isn’t enough. Plenty of companies keep pushing the importance of routine hazard assessments and protective measures, but accidents still happen. Every chemical, no matter how obscure, leaves behind a paper trail—of spills, burnt-out gloves, or unexpected fumes. I’ve seen even seasoned researchers underestimate not-so-famous chemicals until near-misses force a sharp reassessment.
Neglecting the risks from 2,3-Phenazinediamine could lead to employee health issues or environmental contamination. Waterways near industrial plants are sometimes the final resting place for chemical runoff, and as we’ve learned from history, weak wastewater controls have poisoned groundwater and harmed communities.
Companies that work with 2,3-Phenazinediamine, even infrequently, owe their workers more than just a warning sticker. Adequate ventilation does more than keep the air fresh—it tips the balance from risky to responsible. Gloves, goggles, and long sleeves are non-negotiable gear. Rinsing skin quickly after a spill can make the difference between a short-lived sting and a lasting rash. If waste management is sloppy, local water supplies might take the hit, so sealed containers and prompt disposal carry real weight.
Researchers and chemical users can pressure suppliers for clearer hazard information. We tend to trust the data sheets are complete, though some gaps still exist. Pushing for more transparent studies on health and environmental impacts can drive future safety standards. Anyone involved should learn the real risks, not the ones guessed at around breakroom coffee. A culture of curiosity, paired with daily vigilance, strengthens the safety net for everyone involved—from factory workers to downstream communities.
Regulations work only as hard as the people enforcing them. The more we demand clear labeling, proper storage, and open records, the less likely we are to look back with regret after preventable accidents. That’s why reading, thinking, and speaking up about chemicals like 2,3-Phenazinediamine still matters, long after the lab lights shut off.
| Names | |
| Preferred IUPAC name | 3,4-Diaminophenazine |
| Pronunciation | /ˈfiː.nəˌziːn.daɪˌæm.iːn/ |
| Identifiers | |
| CAS Number | 492-62-6 |
| Beilstein Reference | 120924 |
| ChEBI | CHEBI:38485 |
| ChEMBL | CHEMBL156361 |
| ChemSpider | 167359 |
| DrugBank | DB13157 |
| ECHA InfoCard | 17ccf588-f190-4a8a-9182-cd8e82c7ec90 |
| EC Number | EC 203-712-6 |
| Gmelin Reference | 6810 |
| KEGG | C07075 |
| MeSH | D011981 |
| PubChem CID | 86166 |
| RTECS number | SS9750000 |
| UNII | 0O1M5Q577M |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | DTXSID8035833 |
| Properties | |
| Chemical formula | C12H10N4 |
| Molar mass | 208.24 g/mol |
| Appearance | Gray to brown solid |
| Odor | Odorless |
| Density | 1.32 g/cm3 |
| Solubility in water | slightly soluble |
| log P | 0.4 |
| Vapor pressure | 1.87E-4 mmHg at 25°C |
| Acidity (pKa) | 5.21 |
| Basicity (pKb) | 5.15 |
| Magnetic susceptibility (χ) | -62.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.760 |
| Dipole moment | 2.72 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 146.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -3.2 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -52.8 kJ/mol |
| Pharmacology | |
| ATC code | D08AX02 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause an allergic skin reaction. Suspected of causing genetic defects. Suspected of causing cancer. |
| GHS labelling | GHS05, GHS07, GHS08 |
| Pictograms | GHS07,GHS09 |
| Signal word | Danger |
| Hazard statements | H302, H317, H319, H334, H341, H350 |
| Precautionary statements | P264, P270, P273, P280, P301+P312, P302+P352, P305+P351+P338, P308+P313, P330, P337+P313 |
| Flash point | Flash point: 243°C |
| Autoignition temperature | 363 °C |
| Lethal dose or concentration | LD50 oral rat 237 mg/kg |
| LD50 (median dose) | LD50 (median dose): 2370 mg/kg (rat, oral) |
| NIOSH | PH8225000 |
| PEL (Permissible) | PEL: 0.1 mg/m³ |
| REL (Recommended) | REL: 0.1 mg/m³ |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Phenazine Neutral red Safranin Phenazine methosulfate |