Chemists became interested in pyrazines not long after organic chemistry blossomed in the nineteenth century. Before lab coats had zippers, early researchers isolated pyrazines from coal tar and noticed their place in roasted and cooked aromas. By the mid-1900s, the food industry stumbled upon 2,3-dimethyl pyrazine while poking around in coffee and cocoa studies. This little molecule left a big mark on the world of flavors. Its nutty, roasted character first drew attention for use in food, especially once scientists figured out how to make it reliably in big batches instead of relying on extraction from plants or foods.
When you meet 2,3-dimethyl pyrazine in a lab, you don’t forget the scent. It brings the memory of toasted bread or warm nuts to your nose. This chemical often finds itself in a clear or pale yellow liquid form, packed tightly in sealed glass, away from sunlight and air. Flavorists in food and perfume labs, and even chemists in the electronics sector, look to 2,3-dimethyl pyrazine for its stability and consistency in taste and aroma. Large-scale suppliers track purity levels using gas chromatography, because even small traces of other chemicals can throw off the subtle smell.
2,3-Dimethyl pyrazine stands out because it weighs just 108.15 g/mol, stays liquid just above room temperature, and boils at roughly 170°C. The density hovers around 0.98 g/cm³. The molecule doesn’t mix well with water but dissolves into alcohols and organic solvents like a charm. Just a few parts per billion make food taste roasted or nutty, which means a little goes a long way. Its chemical backbone—a pyrazine ring with two methyl groups stuck on the 2 and 3 positions—makes it stable, even under gentle heating, which suits larger manufacturing setups.
Manufacturers slap high purity numbers on bottles: 98% and higher for food-grade and 99% up for laboratory or electronics work. Typical labels call out CAS number 5910-89-4, molecular formula C6H8N2, and standardized hazard statements. Every drum or bottle should include a batch number, net weight, and recommended storage: cool, dark, and dry. Safety sheets flag its flammability and mild irritant potential, so proper gloves and eye protection become part of the drill on the shop floor.
Most 2,3-dimethyl pyrazine on the market today comes from chemical synthesis—specifically, by cyclization of the right alpha-diketone (like 2,3-butanedione) and a reactive amine (often 1,2-diaminopropane). Reaction conditions have to be dry and oxygen-free for the best yields. Catalytic or thermal cyclization brings the ring together and precision matters here: stray water or air leads to breakdown or useless byproducts. For labs without big reactors, small-scale prep isn’t too tricky, but strict control of temperature and reagent ratios keeps the final product pure and sticky hands to a minimum.
In the hands of a skilled chemist, 2,3-dimethyl pyrazine reacts in predictable ways. Halogenation, nitration, or alkylation tweak the pyrazine core, sometimes giving new scents or flavors. The methyl groups on the ring act as shields, which means modifications go for open spots on the ring or poke through with strong catalysts. In research settings, folks like to attach functional groups for sensors, dyes, or flavor analogs, and the robust skeleton resists breaking down during these modifications.
This chemical responds to a bunch of aliases—2,3-dimethylpyrazine, Pyrazine, 2,3-dimethyl-, FEMA 3273, and its EINECS registry number, 227-680-0. Global suppliers may tag it under food flavor enhancer lists or within aroma ingredient catalogs. Every marketplace, from EU to USA to East Asia, uses these identifiers to cut through language barriers and avoid mix-ups on global shipping manifests.
Working with volatile organic compounds like this one means following basic safety rules. Good ventilation, flame-proof storage, gloves, and goggles keep people safe. On the shop floor or in pilot plants, employees check for vapor leaks and treat spills with absorbent pads and proper disposal. Regulatory agencies such as OSHA or Europe’s REACH have clear documentation for storage and transportation, and every major supplier includes a material safety data sheet. Since the odor threshold is low, even small spills alert staff quickly. Plus, because it doesn’t play nice with strong oxidizers, facilities check compatibility before stacking chemical drums.
Food flavoring steals most of the limelight for 2,3-dimethyl pyrazine. Coffee, cocoa, roasted nuts, baked goods, and even grilled meat products benefit from the whiff of roasted, nutty aroma it brings. Snack manufacturers use this chemical to mimic expensive natural roasting when margins tighten. In the world of perfumery, it sits behind warm, toasty notes. Some electronic material engineers value its stability, using it in specialized organic synthesis or in the design of new sensor materials. In agriculture, researchers explore its role as a plant signaling molecule, aiming for eco-friendly pest control. It pops up in librations of emerging biotech, too, as part of biosensor and bioreactor advances.
Scientists keep poking at the question: Can microbes make 2,3-dimethyl pyrazine better than chemists do? Some labs tinker with engineered yeast or bacteria to snatch value from food processing waste, spinning it into high-purity chemical feedstocks. Across academic journals, you’ll find studies on its interaction with taste receptors, its breakdown in complex flavor matrices, and new synthetic pathways using greener or softer reaction conditions. Analytical chemists hunt for faster, cheaper ways to measure trace levels in food products, especially as consumer watchdogs demand tighter scrutiny in labeling.
On the safety front, animal studies flag a low acute toxicity for this compound. At normal levels in food, it stays within flavor safety guidelines set by groups like FEMA (Flavor and Extract Manufacturers Association) and JECFA (Joint FAO/WHO Expert Committee on Food Additives). Some inhalation studies in mice report nose and throat irritation at high concentrations, but daily exposure in food remains well below those thresholds. Still, long-term chronic exposure hasn’t been studied as thoroughly, and the chemical industry keeps one eye on possible allergic reactions or cumulative effects in workers who handle it often. Regulatory agencies continue to evaluate fresh data as it comes.
With plant-based and alternative proteins going mainstream, demand for natural, roasted, meaty flavors is growing. Foodtech startups hope biotechnological methods can scale enough to meet demand while sidestepping petrochemical origins. At the same time, sensor engineers see opportunities in electronics and environmental monitoring using these stable aromatic compounds. For workers and communities near production sites, green chemistry and zero-emission production form the next big wish. Academic research may unlock biochemical pathways for making related flavor compounds cheaper, or find entirely new uses for this sturdy little molecule in materials science and medicine. All signs point to a future where 2,3-dimethyl pyrazine impacts flavor and tech in new, unexpected ways.
Open a bag of roasted coffee or fresh-baked bread. The rich, slightly nutty scent hits you straight away. That signature note comes in part from a compound called 2,3-Dimethyl Pyrazine. It shapes flavors not just in coffee, but across a huge range of products. Companies in food and beverage industries often seek out this molecule to mimic or intensify roasted, nutty, and earthy notes in everything from chocolate bars to snack mixes. Many people would be surprised to learn that the same essence filling bakeries can be found in processed food ingredients.
Commodities like cereals and snacks rely heavily on 2,3-Dimethyl Pyrazine. Without it, artificial flavors tend to taste hollow or flat. Real food has layers, built up by many compounds working together. 2,3-Dimethyl Pyrazine punches above its weight, bringing depth to even the most basic snacks. Food technologists chased after the perfect simulation of roasted peanuts or grilled burgers, landing on this compound after much trial and error. Less can definitely be more: even in tiny doses, the compound tweaks a bland formula into something rich, warm, and memorable.
Stepping outside the kitchen for a moment, perfumers and fragrance developers borrow from the same chemistry. 2,3-Dimethyl Pyrazine infuses complexity into perfume bases. Used subtly, it brings a cozy familiarity to a scent, often evoking comfort or nostalgia. A whiff of this compound immediately connects the brain to experiences around food, warmth, and togetherness, which marketers recognize and harness in home products.
Widespread use sometimes raises eyebrows, especially as synthetic additives come under heavier scrutiny. Luckily, regulatory bodies such as the U.S. Food and Drug Administration give 2,3-Dimethyl Pyrazine the green light as a food additive. Researchers also continuously monitor its safety profile. There’s solid evidence pointing to its safety in the low doses used in flavoring.
Many shoppers now scan ingredient lists, looking for recognizable sources. “Natural” and “clean label” trends throw a spotlight on compounds like 2,3-Dimethyl Pyrazine. Consumers often assume synthetic-sounding names mean artificial everything, even though the substance occurs in real roasted foods. Manufacturers have started pivoting toward natural extraction, sometimes using microbial fermentation or roasting plant-derived starting materials. Sourcing the compound naturally satisfies both regulatory requirements and growing consumer expectations.
With plant-based and alternative proteins gaining ground, trustworthy flavor becomes even more important. New products need all the help they can get to win over skeptical eaters, and 2,3-Dimethyl Pyrazine proves its worth by helping alternative meats and cheeses taste just right. There’s an ongoing race to balance taste, safety, and consumer trust. It always comes back to that familiar, irresistible aroma and flavor that reminds us why food traditions stick around in the first place. For anyone interested in how flavor science works behind the scenes, this unassuming little molecule gives plenty to chew on.
Walking through any flavor chemistry lab, certain numbers keep popping up on bottles: 98%, 99%, sometimes an eye-catching 99.5%. This isn’t just a marketing move. Most of the 2,3-dimethyl pyrazine available from chemical suppliers arrives at about 98% purity or higher. In the flavor and fragrance world, that extra percent or two can make an outsize difference.
From my own experience helping a local food producer with regulatory paperwork, hitting that high purity isn’t just about avoiding side-eye from regulators. Regulatory bodies like the FDA are strict about what ends up in the food supply. A 98% purity means only two grams out of every hundred are something else. In a finished flavor, that could change a formula’s safety profile or leave room for off-tastes. The last thing a chocolatier wants is to find their new bar suddenly has notes of something far from cocoa, all because of an impurity floating around at a concentration no one checked.
At a sensory level, I remember a time when a batch of roasted-nut flavored snacks felt just a little “off.” It turned out the issue traced back to a pyrazine with purity under 96%. That missing couple of percent really did twist the warm, nutty aroma toward something almost musty. No marketing spin could fix that.
Producing 2,3-dimethyl pyrazine isn’t a backyard chemistry project. Typical syntheses throw off a mix of related compounds—some accidentally boost the roasted or earthy notes, others don’t help at all, or might even be flagged as food safety concerns. Purification steps drive up both cost and environmental impact. I’ve talked to plant engineers who weighed whether to keep running extensive chromatography cycles just to raise purity from 98% to 99%—all for a product priced tightly by the kilogram. If the end use is in trace flavoring, most users stick with 98% and carefully review what’s in those other two grams.
Most big-name suppliers list 2,3-dimethyl pyrazine in the 98–99% range, sometimes offering higher-purity lots for medical or analytical use. The baseline figure isn’t chosen at random. Regulations for food-grade additives set strict upper limits on certain heavy metals, solvents, or residual reactants, and those last bits get monitored before every shipment leaves a warehouse. Even with those protections, cost-sensitive buyers sometimes wonder if 97% would do the trick, especially since margins on bulk production are slim.
Online catalogs sell to research labs at higher purities—think 99.5% and up—but the jump can double or triple the price per gram. Scale that up to commercial batches, and you see why most factories settle in the high nineties for daily work.
Some producers invest in continuous purification processes, cutting waste and energy while aiming for higher-purity ingredients at a reasonable price. Improving synthesis chemistry to give fewer unwanted byproducts solves problems at the source, rather than after the fact with heavy purification. As taste science advances, the tolerance for minor impurities gets even smaller, so industrial plants keep tweaking their process controls.
For anyone considering a new supplier or a batch of 2,3-dimethyl pyrazine that’s significantly under 98%, proceed with caution. Even tiny chemical distractions can show up in flavor or safety tests. As the bar keeps moving higher, quality matters as much as ever.
2,3-Dimethyl Pyrazine often pops up behind the scenes in snack and flavor industries. This chemical packs a powerful punch for such a small molecule. Storing it takes a few practical steps. Any time I’ve worked around strong-smelling, volatile compounds like this one, air-tight containers became my best friends. Glass bottles with proper closures cut down on evaporation and odor transfer—a real lifesaver for anyone who doesn’t want their whole storage room smelling like roasted nuts or popcorn.
Leaving bottles unsealed encourages the aroma to spread, which isn't just unpleasant—it welcomes spoilage and loss of valuable material. One batch I saw poorly closed didn’t just lose potency; it made an entire shared shelf unusable for weeks. Storage at room temperature tends to work if the environment stays dry and cool. Chemical stability takes a hit if the place gets too hot or damp. So, I always aim for a spot that avoids direct sunlight, away from heat sources or windows.
People sometimes forget that even flavor compounds deserve respect in the lab or plant. Gloves, goggles, and good ventilation make a huge difference. More than once, I’ve seen someone underestimate a compound like this because of its small volume. That’s a fast way to experience throat or eye irritation, since the vapor is a little sneaky. If you spill any, a damp cloth traps vapors quickly—instead of sweeping them up and pushing more into the air.
I always promote storing chemicals on lower shelves to avoid breakage if something tips over. Keep food and drink away from storage or work areas. The International Agency for Research on Cancer keeps 2,3-Dimethyl Pyrazine off its big risk lists, but that doesn’t mean tossing aside good habits. It only takes one mistake to contaminate food prep spaces.
Flammable vapors don’t play around, and 2,3-Dimethyl Pyrazine fits that description. I remember a coworker once uncapped a similar compound too close to a hot plate. A brief sizzle reminded us to use designated, spark-free storage cabinets for all flammable organics, and to keep fire extinguishers handy. Local fire codes sometimes get ignored, but regular quick checks on expiration, label clarity, and cap fit beat any headache later.
Handwriting fades. Labels get smudged. Chemicals end up decanted without names or dates. Lots of folks think memory is good enough, until it isn’t. I got called in years ago to identify an “unknown brown bottle” that turned out to be harmless, but it could just as easily have been something dangerous. Always mark the name, concentration, date received, and intended use, straight on the container. Nobody likes mysteries in chemical storage.
Local rules handle chemical waste in unique ways. Dumping leftovers or rinsing glassware in the sink causes more trouble than it saves. I keep sealed waste containers, log entries, and always partner with licensed disposal services—even if it takes a day or two longer. Accidental release into sewer systems ends up costing more later, whether it’s in cleanups or regulatory fines.
2,3-Dimethyl Pyrazine isn’t the most dangerous substance around, but every time someone takes a shortcut in its storage, handling, or labeling, risk creeps up. Consistency, clear labeling, and clean separation from food zones go further than any warning label. In the end, good handling rests on personal routines, not just policy handbooks.
You’ve probably never wondered what gives roasted nuts, grilled steak, or toasted bread that nutty, mouth-watering aroma. Most folks chalk it up to “the magic of cooking.” The real kicker is that compounds like 2,3-Dimethyl Pyrazine handle much of the heavy lifting. This piece isn’t just for food chemists. Anyone who’s ever been tempted by a chocolate bar’s smell or dreamed of biting into crispy fried onions bumps into pyrazines every day, whether they know it or not.
So, is it safe to put this stuff in actual food flavors? The short answer: If 2,3-Dimethyl Pyrazine comes with food grade certification, it’s considered safe for use in flavors and fragrances. Food safety rules aren’t just empty talk. Every batch has to clear hurdles set by organizations like FEMA (Flavor and Extract Manufacturers Association) and the FDA. They both keep an eye on how much is used and how the ingredient is sourced. In the U.S., FEMA GRAS status has been granted for 2,3-Dimethyl Pyrazine, meaning it’s “Generally Recognized As Safe” for flavor use under specific conditions. That’s a good sign for cooks, bakers, and snack-makers.
Pyrazines like this one don’t show up in giant spoonfuls. The actual quantities in foods are measured in parts per million. A tiny bit goes a long way. In all those crispy, caramelized notes, it takes just a flicker of this compound to make a real difference in taste. There’s even a cottage industry built around blending these molecules into everything from ice cream to potato chips.
Just because something can be found in nature doesn’t mean it’s always a sure bet for dinner. As someone who grew up in a family that wouldn’t let anything questionable touch the dinner table, I get the urge to double-check ingredients. Food grade supply isn’t about chemical structure so much as it’s about purity and handling. A lab could churn out 2,3-Dimethyl Pyrazine that is technically identical to what’s in roasted coffee but cut corners with contaminants during production. That’s the kicker: certification proves the product was made, stored, and shipped with edible safety in mind.
Folks working in flavor labs know these safeguards aren’t just for show. Without careful vetting of suppliers, the risk of impurities jumps. It’s worth reminding those who blend and bake for a living: food grade isn’t a box-tick; it’s the difference between a safe product and a product that causes recalls.
Many food flavor companies work with global supply chains these days. That means more chances for mistakes or quality drops. Countries sometimes disagree on what’s okay in food. Setting international standards for food grade pyrazine production, with transparent tracking and testing, would help. Build better trust with clearer labels, and the whole system gets safer.
Curiosity about what’s really in your favorite snack isn’t just interesting talk—it’s protection. Anyone who cares about what goes into food, whether they’re making it or eating it, deserves honest, documented safety. 2,3-Dimethyl Pyrazine brings a lot to the table, but it pays to check the paperwork before taking a bite.
Stepping into a lab or a food production site, it’s easy to notice that chemicals like 2,3-Dimethyl Pyrazine don’t come in neat, one-size-fits-all packages. In my early years working with flavor compounds, I quickly learned that packaging isn’t just an afterthought. It shapes how people use an ingredient and how wasteful or efficient a process becomes.
2,3-Dimethyl Pyrazine usually shows up in bottles or drums. Most suppliers keep the range practical. I’ve seen glass bottles as small as 5 grams, often used in research and development labs, where every penny and drop gets counted. For flavor houses or tobacco manufacturers, 100-gram, 250-gram, and 500-gram amber bottles seem to hit the sweet spot. You can grab just enough for a batch or small project and move on without unnecessary leftovers eating up shelf space.
Walk deeper into industrial territory, and drums take over. Ten-kilo and 25-kilo metal or HDPE drums appear behind factory gates. Here, nobody wants to waste time unsealing tons of little bottles. Safety, cost, and convenience point directly at bigger containers, especially for firms that blend or process tons daily.
Most people think about cost-per-unit and leave it at that. But waste looms large. In small labs, an oversized bottle of 2,3-Dimethyl Pyrazine can mean you end up tossing expired material. I remember working with a beverage company that ordered by the kilogram for months — only to toss more than half. Smaller 50-gram or 100-gram bottles would’ve prevented spoilage and cut down their hazardous waste bill.
Flip the coin, and big plants can’t afford to waste time fussing over cap after cap. They profit from bulk, so a few sturdy drums make more sense. Fewer empties, fewer order delays, fewer headaches.
Each size comes with baggage. Small bottles mean more hands-on time — every transfer brings a new spill risk. With glass bottles especially, running a busy shift can lead to an accident. I’ve nearly lost a few small samples by the simple act of fumbling with a cap while juggling gloves and notes. Bulk drums pose other risks, particularly with volatile chemicals. Once unsealed, a 25-kilo drum demands quick, careful handling and strict storage rules to keep the compound fresh.
Instead of a standard answer, I find it better to discuss the work itself. What works for a university research group — small, well-labeled glass bottles — looks completely different from the blunt practicality of a food processor’s workflow. In my experience, the best results happen when folks actually talk to their chemical supplier about batch size, timeline, handling, and storage quirks. Some suppliers now offer customized fills, meeting halfway between the usual sizes, which bridges the gap.
Simple steps like switching from glass to plastic for transport, or using smaller fills within a bulk shipment, have saved teams I’ve worked with time and money, not to mention fewer emergencies come audit season.
In the end, picking the right packaging for 2,3-Dimethyl Pyrazine can lower total cost, keep workers safer, and cut back on waste. People facing these choices every day know the value of sizing up their real needs before signing off another order form.
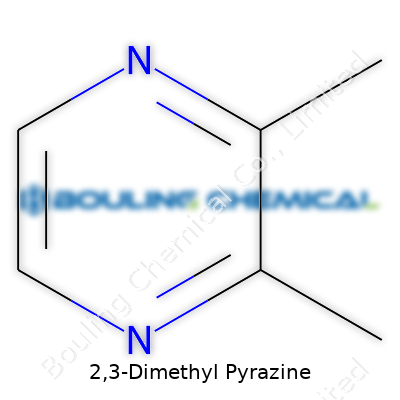
| Names | |
| Preferred IUPAC name | 2,3-Dimethylpyrazine |
| Other names |
2,3-Dimethylpyrazine Pyrazine, 2,3-dimethyl- 2,3-Dimethyl-1,4-diazine |
| Pronunciation | /tuː θri daɪˈmɛθɪl paɪˈræziːn/ |
| Identifiers | |
| CAS Number | 5910-89-4 |
| 3D model (JSmol) | CS1=NC=CN=C1C |
| Beilstein Reference | 166516 |
| ChEBI | CHEBI:34685 |
| ChEMBL | CHEMBL373055 |
| ChemSpider | 67571 |
| DrugBank | DB14016 |
| ECHA InfoCard | 07a7fa56-5e48-44f4-9b4b-2c943cbb1f48 |
| EC Number | 211-816-8 |
| Gmelin Reference | 7877 |
| KEGG | C09879 |
| MeSH | D016603 |
| PubChem CID | 12941 |
| RTECS number | UJ1050000 |
| UNII | D9B49S4NVX |
| UN number | NA3082 |
| CompTox Dashboard (EPA) | DTXSID9020705 |
| Properties | |
| Chemical formula | C6H8N2 |
| Molar mass | Molar mass: 108.14 g/mol |
| Appearance | Appearance: Colorless to pale yellow liquid |
| Odor | nutty; roasted; cocoa |
| Density | 0.948 g/mL at 25 °C (lit.) |
| Solubility in water | Soluble in water |
| log P | 0.41 |
| Vapor pressure | 1.42 mmHg (at 25 °C) |
| Acidity (pKa) | 14.6 |
| Basicity (pKb) | 4.38 |
| Magnetic susceptibility (χ) | -53.6×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.4970 |
| Viscosity | 1.067 cP (at 20°C) |
| Dipole moment | 1.93 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 209.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -13.3 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3459 kJ/mol |
| Hazards | |
| GHS labelling | GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | P261, P264, P271, P273, P280, P301+P312, P304+P340, P305+P351+P338, P332+P313, P337+P313, P362+P364, P501 |
| Flash point | 69°C |
| Autoignition temperature | 230 °C |
| Explosive limits | 1.2–9.2% |
| Lethal dose or concentration | LD50 (oral, rat): 4380 mg/kg |
| LD50 (median dose) | LD50 (median dose): 730 mg/kg (rat, oral) |
| NIOSH | NIOSH DH6650000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 mg/m³ |
| Related compounds | |
| Related compounds |
2,5-Dimethylpyrazine 2,6-Dimethylpyrazine 2-Methylpyrazine 3-Methylpyrazine Pyrazine Tetramethylpyrazine |