The story of 2,3-Dimethyl-5-Ethyl Pyrazine goes back to the mid-20th century, in the era when flavor chemistry started turning heads. Chemists caught on to the molecule while hunting for the source of earthy, roasted notes that trickle through natural aromas. Back then, folks didn’t have fancy machines like gas chromatography-mass spectrometers. They worked off intuition, nose, and a bit of luck. Pyrazines—this class of compounds—began getting real attention because of their contribution to flavors in roasted coffee, baked goods, and even cooked vegetables. Researchers published the first syntheses and analytic measurements in academic journals, and not long after, food scientists began tinkering with them to create more consistent flavors in processed products.
2,3-Dimethyl-5-Ethyl Pyrazine delivers a punchy, nutty, roasted flavor, making it one of the prized pyrazines in the food industry. It manages to sneak into a raft of products—from chocolate bars to potato chips—giving that signature ‘cooked’ aroma you notice in roasted nuts or coffee. Beyond the kitchen, this molecule has a place in fragrance creation, especially for those looking to capture earthy and warm base notes. Industrial suppliers bottle it up in high-purity form, making sure to tailor quantities from tiny vials for research labs to larger shipments for megafactories.
This compound carries the chemical formula C8H12N2. It usually pops up as a colorless to pale yellow liquid. Pyrazines as a group sit in the aromatic heterocycle club, with a ring structure that’s somewhat like benzene but swaps in nitrogen for a couple of carbons. The boiling point hovers around 190-195°C, and it releases that famous aroma even at low concentrations. It isn’t the type to dissolve in water easily; organic solvents do the trick, whether in the lab or industry. Its stability under normal storage means it doesn’t give users too many headaches as long as they keep containers sealed and away from open flames.
Commercial-grade 2,3-Dimethyl-5-Ethyl Pyrazine tends to reach purities above 98%. Producers supply it with complete labeling—chemical identity, batch number, purity, recommended storage temperature, and safety warnings. Some suppliers toss in detailed chromatographic charts for extra peace of mind. Industry regulations in places like the EU and U.S. force everyone to list key hazards, recommend personal protective equipment, and mention environmental considerations. Barcodes and digital tracking allow bulk buyers to keep tabs from warehouse to production line. These details help teams trace and recall products if quality issues pop up.
Synthesis usually follows established chemical routes that date back several decades. One popular recipe involves cyclocondensation between a suitable diketone and a diamine, all under mild heat. Chemists often choose acetoin or a similar ketone, paired with a methyl- or ethyl-substituted diamine to introduce the desired side chains. Industrial facilities tend to automate steps for high yield and purity. Purification often goes through distillation or column chromatography, much like many laboratory-prepared aromatic compounds. Residues and byproducts call for careful disposal to avoid environmental headaches or regulatory run-ins.
2,3-Dimethyl-5-Ethyl Pyrazine does not stand as the most reactive aromatic ring out there—thanks to those nitrogen atoms—but certain side reactions open doors for customization. Halogenation to introduce flavor variations works, so does selective methylation for creating tailored analogs. A few labs run hydrogenation steps to explore saturated derivatives, although this often tames the distinct aroma character. Some researchers dig into electrophilic aromatic substitution, hoping to unlock new variations for both flavor and pharmaceutical studies. Each modification gets run by taste panels and safety reviews before landing in consumer products.
Chemists sometimes call this compound Ethyl Dimethylpyrazine, or just shorthand ‘EDMP’. CAS registry number 25683-07-2 provides an unambiguous tag. Food scientists and ingredient makers toss around labels like roasted flavor concentrate or nutty aroma agent in their supply chain paperwork. Marketing departments get creative for consumer-facing products, dressing up ingredient lists with terms like natural pyrazine or proprietary flavor blend, though regulations demand the right IUPAC or standardized food additive code in formal paperwork and technical sheets.
Handling any aromatic pyrazine calls for smart lab or plant practices. Direct contact with skin or eyes brings risks—think irritation, the kind of burning sensation many chemists remember after a strained glove snaps. Facilities enforce gloves, goggles, and a decent ventilation system. Fire risks stay low thanks to the high flash point, but vapor can catch fire if exposed to strong ignition sources. Regulatory bodies like OSHA and ECHA in Europe require clear labeling with pictograms showing health hazards. Procedures include ventilated hoods, spill kits, and emergency showers—no one wants yet another horror story about unprepared lab crews. Waste streams carrying pyrazine derivatives usually go through chemical neutralization or controlled incineration, keeping the compound out of groundwater and local ecosystems.
Most people brush past flavor chemistry without a second thought, but many favorite foods pick up their special notes thanks to compounds like 2,3-Dimethyl-5-Ethyl Pyrazine. Food manufacturers blast small doses into chocolates, coffee blends, nut snacks, and savory seasonings. Even pet food includes this molecule to make kibble more enticing. Outside the world of snacks and treats, fragrance designers tap it to build complex scent profiles, leaning on its earthy and roasted tones to anchor perfumes. Agricultural scientists sometimes use it as a study tool for tracking odorants that attract or repel insect species. In specialty cases, biotech labs run trials involving the pyrazine to monitor its metabolic fate in plant or microbial biosynthesis pathways.
Lab time never stops for pyrazines. Academic teams keep exploring how 2,3-Dimethyl-5-Ethyl Pyrazine binds to sensory receptors. New detection tools push limits, measuring single-molecule thresholds in foods or packaging materials. Analytical chemists amplify efforts around rapid screening—looking for ways to distinguish real flavors from artificial additions. Some researchers hunt for biotechnological production routes, inserting genes into microbes to generate the compound sustainably rather than relying solely on chemical synthesis. Food scientists scrutinize stability during baking or frying, looking for the sweet spot that keeps flavors potent after processing. Research communities keep trading insights at conference sessions, hoping to inspire safer, greener, better-tasting outcomes.
People want to know whether what they eat or smell can cause harm, so toxicology studies always follow new uses for compounds like this pyrazine. Testing covers acute and chronic exposure—in rodents, cell cultures, even simulated gastric fluids. Most regulatory reviews put 2,3-Dimethyl-5-Ethyl Pyrazine in the low-risk category at the trace levels used in foods. At higher concentrations, issues like mucosal irritation or mild nervous system symptoms can pop up, reminding everyone why personal protective gear matters during handling. Scientists keep an eye out for long-term outcomes, looking at metabolic breakdown, tissue accumulation, and potential allergic responses. Public agencies demand updated safety reviews every few years as ingredient use climbs and detection methods improve.
The world leans into plant-based foods and natural flavors, and that puts renewed spotlight on molecules like 2,3-Dimethyl-5-Ethyl Pyrazine. Consumer trends drive suppliers to look for biosynthetic production, harnessing yeast or bacteria rather than fossil-oil-derived feedstocks. Green chemistry attracts funding, as companies see cost savings and environmental wins from cleaner processes and renewable raw materials. Artificial intelligence and digital modeling promise to map structure-odor relationships, helping developers create even more precise flavor profiles. Future work could see this molecule expanded into therapeutics, insect behavior modification, or sustainable crop management, depending on research priorities and funding. With flavor innovation at the core, demand from both mainstream and niche companies will almost certainly push further refinement, bolstered by ongoing safety evaluations and new approval pathways.
Ask anyone in the food science world and many will tell you: few chemicals carry as much weight with flavorists as 2,3-Dimethyl-5-Ethyl Pyrazine. With a name straight from a chemistry exam, it doesn’t draw much attention from shoppers cruising grocery aisles. But that same tongue-twister plays a quiet role in making grilled meats, coffee, and nuts taste a whole lot better.
Pyrazines crop up naturally after roasting, grilling, or baking. Take a batch of coffee beans—roast them, and the kitchen fills with a deep, earthy, almost roasted-cereal aroma. That’s the magic of pyrazines, including this particular variety. Food scientists rely on this compound to give snacks or ready-made meals a savory punch, especially where cost or convenience rules out traditional roasting.
It’s not just about taste. In my years working next to commercial kitchens, chefs turned to pyrazine-based seasonings when keeping consistent flavor from one day to the next. Nature can be fickle—one crop of peanuts tastes bold, another seems bland. By adding a few drops of 2,3-Dimethyl-5-Ethyl Pyrazine, brands sidestep season-to-season changes and make sure every bag of nuts tastes like the last.
Anyone who’s enjoyed a bag of roasted peanuts or cheese-flavored chips has likely tasted the results of this minor ingredient. Its most common use sits inside snack flavors—think nuts, crackers, barbecued chips, even instant ramen. Companies mix it in low amounts, yet that small touch makes flavors seem rich and roasted, pushing even bland base products closer to something fresh-off-the-grill.
Beyond snacks, you’ll spot it in some coffee blends, especially the types that get labeled "French Roast" or "Dark & Bold." Not every coffee bean brings that smokey, cocoa-kissed note. With judicious use of 2,3-Dimethyl-5-Ethyl Pyrazine, roasters can bridge the gap between different harvests and offer their signature cup year-round.
Questions about food additives come up all the time. People prefer ingredients they recognize, and something like 2,3-Dimethyl-5-Ethyl Pyrazine doesn’t exactly stand out as natural. Regulatory bodies give it the green light in tiny doses, echoing what I’ve seen—too much and the flavor tips from full-bodied to synthetic.
Folks care about what lands in their food, so food manufacturers would do well to provide simple ingredient lists and clear communication. For every technophile wowed by chemistry, there’s a parent who wants to know if something’s safe for their kid. While this pyrazine offers flavor consistency, it shouldn’t be a band-aid for low-quality ingredients. Better farming and transparent sourcing give customers both great flavor and peace of mind.
Flavor chemistry always walks a line—balancing cost, taste, and the stories people want on their tables. 2,3-Dimethyl-5-Ethyl Pyrazine keeps snack foods tasty, but as more people read between the lines, honest labeling and focus on quality will matter just as much as the science inside the package.
Biting into a perfectly toasted peanut, there’s often a crisp roasted zing that sparks nostalgia. Those punchy, savory notes show up in all kinds of cooked food: from dark-crusted bread to fresh-roasted coffee. Much of that magic boils down to a quirky group of molecules called pyrazines. Among them, 2,3-dimethyl-5-ethyl pyrazine stands out for its ability to mimic the smell of just-popped popcorn or browned nuts.
This compound doesn’t leave much room for subtlety. Its signature flavor packs a wallop—think fresh peanut skins, salted cashews, and the oily aroma rising from warm hazelnuts. I still remember the first time I worked with food flavorings in a test kitchen. The tiny drop of pure 2,3-dimethyl-5-ethyl pyrazine transformed bland cereal into something that felt baked and hearty. The smell hit hard—earthy, a little sweet, and unmistakably roasted. People often call it “nutty,” but that understates things. There’s a rich complexity to it—like the browned edges of toasted oats, the bitter bite of burned sugar, and an echo of green bell pepper.
The real beauty comes through when a whiff brings back memories: shelving peanut butter jars at a grocery store, camping on mornings with instant coffee, or snagging a handful of trail mix before a long bus ride. Even small amounts bring out the comfort of roasted nuts or the warmth of a wood-fired oven. Science backs up this sensory experience. A 2022 flavor wheel analysis listed 2,3-dimethyl-5-ethyl pyrazine as a heavyweight for the peanut-like spectrum and flagged it as essential in the aroma of roasted coffee and cocoa.
This molecule is a backbone in the world of snack development. Mass market snacks—think roasted peanuts that taste dialed up, even potato chips that carry a subtle roast—lean hard on it to amplify those flavors. The reason is straightforward: pyrazines don’t just provide aroma, they help trick the tongue into sensing “browned” or “cooked” even in foods that haven’t seen much heat. In my own home baking mishaps, sprinkling in nut-like flavor (instead of browning butter for half an hour) gave quick—and surprising—depth to oatmeal cookies or home-made granola bars.
Cooks and scientists tug at the same problem: it’s tough to fake that roasted flavor naturally. Roasting nuts, bread, or coffee launches these compounds into the air, but only in tiny flashes. Synthetic pyrazines sidestep this, giving food developers a way to bottle up that flavor and splash it exactly where needed.
Some people worry about food flavorings, especially those born in a lab. The truth is, these pyrazines occur naturally during many everyday cooking processes. The roasted notes release when grilling meat, baking bread, or toasting nuts, though the synthetic version cranks up the dial. The bigger issue comes down to honesty: consumers deserve clear labeling. Folks allergic to nuts might feel uneasy seeing “nutty” flavors show up in foods that use no real nuts at all.
One thing that always stuck with me from both shop work and kitchen testing: flavor shortcuts can build craveable snacks. Real progress happens when food companies balance the use of pyrazines with transparency. Ingredient panels with plain language—“contains nut flavoring, not real nuts”—give people the facts and the comfort of knowing what’s really in the bag. There’s no escaping the power of roasted, nutty flavors. With careful use, 2,3-dimethyl-5-ethyl pyrazine can deliver that warmth, just as long as everyone stays honest about what’s inside.
Most people haven’t heard the term 2,3-Dimethyl-5-Ethyl Pyrazine tossed around at dinner. But if you’ve ever enjoyed a toasted snack or opened up a box of breakfast cereal, you’ve tasted the subtle work of this chemistry marvel. Food labs love using this molecule to deliver those warm, roasted, nutty notes to our favorite treats. The question hanging over the kitchen counter: does adding such a substance make our food any less safe?
I’ve spent years working with ingredient labels, and 2,3-Dimethyl-5-Ethyl Pyrazine pops up occasionally, listed under “flavoring.” This compound forms in natural ways, like when coffee beans roast or popcorn heats up. Manufacturers synthesize it in bigger batches, since taste testing proves people love that hint of toasted almond. It’s all about enhancing experience, not tricking your palate — anyone who’s tasted plain rice cakes knows how bland things get without seasoning.
Food scientists run tests to figure out what amounts work and where too much lands. The Flavor and Extract Manufacturers Association (FEMA) and authorities such as the FDA review these chemicals. As of now, 2,3-Dimethyl-5-Ethyl Pyrazine holds FEMA GRAS status. “Generally Recognized As Safe” sounds comforting, but what does that mean for real-world eating?
The safety process for flavors involves toxicity tests on animals, chemical breakdown studies, and monitoring consumption patterns. Decades of data never raised major red flags for small, food-level quantities. I looked up a few studies and saw researchers giving rodents massive doses — more than anyone would ever eat in a lifetime — and still finding no alarming health effects. But these tests don’t cover every long-term risk or unusual sensitivity.
It’s easy to trust review boards, yet there’s always that thought in the back of a shopper’s mind. Food history shows plenty of cases where additives once approved later landed on banned lists. Everyone remembers the old days of additives like Red Dye No.2 or cyclamate sweeteners. Every system leaves room for error, especially when consumer habits shift or science uncovers new findings.
Roasted nuts, baked bread, even chocolate — all of these pack their own pyrazines developed from heat. Regular home cooks don’t fret over chemicals formed in their ovens. The trouble starts when laboratories concentrate these flavors and add them to a vast array of foods. That’s where dosage matters, and constant consumption across various products pushes exposures higher.
Everyday shoppers deserve clear labels, not vague phrases. “Artificial flavor” covers a multitude of ingredients, from everyday vanillin to obscure pyrazines. Many folks want transparency — not out of paranoia, but from a simple right to know what goes into their meals. Small steps could help, like voluntary databases or QR codes on packaging opening up ingredient facts.
Industry and regulators can go a step further. Long-term studies, not just acute exposure checks, help answer questions about routine diets. Sometimes, the safest bet comes from avoiding overload. Eating a wide variety of foods instead of sticking to hyper-flavored snacks keeps intake balanced. I like a grilled sandwich as much as the next person, but it doesn’t take much chemical enhancement to make bland food taste decent.
People crave reliable answers, but science evolves. At the store, I try to buy with my eyes open, choosing fewer processed foods when possible. Not everyone shares the same comfort level with synthetic additives, and that’s fine. As long as companies give us the facts and regulators keep testing, we can decide for ourselves.
2,3-Dimethyl-5-Ethyl Pyrazine might sound like a mouthful, but in many industries—especially in flavor creation—it’s the secret behind that nutty, roasted note you catch in snacks, coffee, and even some bread. I still remember the first time I walked into a development lab and got a whiff of this compound mixed into a simple cracker. It brought out a roundness and warmth I never thought a single molecule could deliver. But just like salt, too much can turn a good thing into a problem—think bitterness, off-notes, or even overwhelming the other flavors you’re working so carefully to develop.
To get technical, most food technologists use 2,3-Dimethyl-5-Ethyl Pyrazine at ultra-low concentrations. Typical usage falls in the range of 0.01 to 1 part per million (ppm)—that’s one milligram in a thousand kilograms of product, at the top end. Some sources recommend staying below 0.2 ppm for subtler foods; for robust profiles, like coffee or barbecue-flavored chips, it can go up to 1 ppm or occasionally a little above. Very few recipes touch that upper limit, and for good reason. Just a bump above the right mark and the taste becomes harsh, even metallic.
This stress on low dosage doesn’t come out of nowhere: pyrazines in general, and this one in particular, pack an intense aroma. Sensory panels in various research found the human nose can spot this compound at just 0.01–0.05 ppm. This pushes food developers to test blends carefully, going drop by drop, often in controlled environments. In professional kitchens, seeing researchers nervously dotting flavor into a sauce grain by grain isn’t rare—they’ve learned from experience.
What about food safety? Both the US Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA) deem 2,3-Dimethyl-5-Ethyl Pyrazine generally safe as a flavoring agent. Restrictions show up only at high doses, far above what’s practical for food use. Chronic toxicity data is limited, but based on structural similarity to other pyrazines, regulators keep tabs for anything new. Some countries may have stricter thresholds on imported foods, but the science lines up: stick to very low ppm, and you’re within recommended safety and sensory margins.
Even small mistakes can be expensive here. In one project I worked on, a junior technician misread a decimal point, spiking a small batch of potato snacks to 5 ppm—no amount of salt or garlic could cover up the sharp, burnt aftertaste. It turned into a lesson in checking, re-checking, and labeling bottles with bold, impossible-to-miss warnings. In production environments, batch sheets and scale checklists have become mandatory not out of bureaucracy but out of repeated, expensive errors.
One solution comes from technology. Modern industrial dosing pumps now handle microgram quantities with near-perfect precision—lessons from pharmaceuticals filtering into the flavor world. Automation, backed by audits, makes mistakes less likely. For the home scale or craft producer, relying on pre-diluted solutions (like dissolving the compound in ethanol first) offers a simple trick: it’s much easier to dose by volume than by weighing tiny crystalline grains.
At the end of the day, 2,3-Dimethyl-5-Ethyl Pyrazine isn’t about chasing the “big flavor.” It’s about using restraint. The real mastery isn’t only in sourcing the purest compound—it’s in having the patience (and humility) to use less than you think you need, to let the roasted notes lift the dish without knocking out everything else. That’s something I had to learn by experience, and it’s still the best advice I’ve ever gotten in the world of food chemistry.
2,3-Dimethyl-5-Ethyl Pyrazine, often known for its strong nutty and roasted aromas, plays a big role in the flavor and fragrance world. I’ve worked with food chemists who use it to sharpen up the taste in chocolate and nuts. What I learned from those folks: this ingredient is more delicate than it seems. One slip in storage, and the profile changes. All that work to get just the right scent goes out the window.
Leaving this kind of pyrazine near sunlight, extra heat, or open air means flirting with faster breakdown and flavor loss. Even a little moisture can do real damage. I remember a time our lab left a container on a sunny countertop for only a week—big mistake. The material arrived rich and warm, but by the time we sniffed it again, the sharp, roasted punch turned faint and odd.
Keeping things tight and cool isn’t rocket science, but it’s easy to cut corners. Once, I watched a team rely on regular warehouse storage in summer. They lost thousands of dollars in aroma compounds to that forgetfulness.
Storing this pyrazine in sealed glass bottles or food-safe metal cans blocks most trouble. Good closure stops exposure to oxygen and water vapor. Avoid plastics that leach smells—they can mess with the pyrazine’s clean signature. I always go for amber glass bottles since they block out sunlight and handle most chemical reactions.
Any time I’ve seen these bottles stored between 5°C and 25°C on a dark, dry shelf, the aroma stayed true. Below zero makes pouring and blending harder and doesn’t really add to longevity. Temperatures above 30°C whip up reactions inside the bottle, shifting aroma and color.
In busy settings, mistakes happen. I’ve seen mismarked bottles cause all sorts of confusion. Sticking with a standard habit—clear labels, open date, and a use-by tally—makes tracing problems much easier. It sounds basic, but once we started logging every batch, the waste almost vanished. Scientists can lean on their fancy lab tools, but for most shops or labs, a dated sticker and logbook work wonders.
This chemical isn’t wine or fine whiskey—aged doesn’t mean better here. Smaller, frequent orders beat buying in bulk and risking stale product. I used to convince management to cut big orders, but after we pitched two overdue drums, they agreed: fresh shipments make smarter business.
Routine housekeeping—quickly checking caps, wiping spills, and avoiding nearby strong-smelling solvents—protects the quality every bit as much as fancy equipment does. One accidental mix-up with a strong cleaning agent once ruined a whole shelf of aroma ingredients. The clean-up took days, and nobody’s forgotten that lesson.
Every time pyrazine lands in a new kitchen or lab, someone counts on its aroma making an impact. Smart storage guards that punchy roast and nut trait. The battle against lost flavor isn’t won with technology but with habits built over time—good bottles, cool shelves, careful labeling, and fresh stocks. Knock those pieces into place, and the rest takes care of itself.
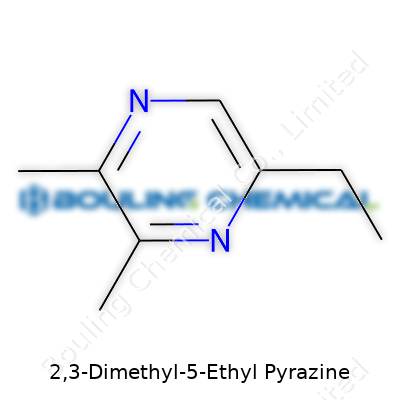
| Names | |
| Preferred IUPAC name | 5-ethyl-2,3-dimethylpyrazine |
| Other names |
2,3-Dimethyl-5-ethylpyrazine 2,3-Dimethyl-5-ethyl-1H-pyrazine 5-Ethyl-2,3-dimethylpyrazine |
| Pronunciation | /tuː θriː daɪˈmɛθɪl faɪv ˈɛθɪl paɪˈræziːn/ |
| Identifiers | |
| CAS Number | 19755-38-7 |
| Beilstein Reference | 110553 |
| ChEBI | CHEBI:133395 |
| ChEMBL | CHEMBL253382 |
| ChemSpider | 163990 |
| DrugBank | DB14136 |
| ECHA InfoCard | 03c9d70d-507a-4123-8e3f-56c264928956 |
| EC Number | 209-642-2 |
| Gmelin Reference | 84459 |
| KEGG | C16433 |
| MeSH | D056709 |
| PubChem CID | 13227339 |
| RTECS number | UJ6725000 |
| UNII | R031OEC822 |
| UN number | NA-UN1265 |
| Properties | |
| Chemical formula | C8H12N2 |
| Molar mass | 166.24 g/mol |
| Appearance | Colorless to light yellow liquid |
| Odor | nutty, roasted, popcorn |
| Density | 0.969 g/cm3 |
| Solubility in water | Insoluble |
| log P | 2.28 |
| Vapor pressure | 0.178mmHg at 25°C |
| Acidity (pKa) | 14.6 |
| Basicity (pKb) | pKb = 9.33 |
| Magnetic susceptibility (χ) | -61.0e-6 cm³/mol |
| Refractive index (nD) | 1.488 |
| Viscosity | 1.081 cP (20°C) |
| Dipole moment | 0.85 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 389.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | −40.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3888.7 kJ/mol |
| Hazards | |
| GHS labelling | GHS07, GHS09 |
| Pictograms | Flame |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P280, P303+P361+P353, P305+P351+P338, P370+P378 |
| Flash point | 54 °C (closed cup) |
| Autoignition temperature | 550 °C |
| Lethal dose or concentration | Lethal Dose (LD50, Oral, Rat): 460 mg/kg |
| LD50 (median dose) | LD50 (median dose): 460 mg/kg (rat, oral) |
| NIOSH | K0163218 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.5 mg/m³ |
| Related compounds | |
| Related compounds |
2,3-Dimethylpyrazine 2,5-Dimethylpyrazine 2-Ethylpyrazine 2,3,5-Trimethylpyrazine 2,3-Diethylpyrazine 3-Ethyl-2,5-dimethylpyrazine 2-Methylpyrazine |