From the heyday of flavor chemistry in the twentieth century, scientists kept their focus on the family of pyrazines for a simple reason: these compounds capture the essence of roasted, nutty, and cooked notes central to foods and scents people crave. Looking back, chemists first isolated pyrazines in the late 1800s, but the branching into specific substituted forms like 2,3-diethyl pyrazine picked up speed in the postwar era. As food processing shifted toward larger industrial scale, labs like those at Firmenich and Givaudan built up rosters of aroma ingredients—2,3-diethyl pyrazine included—mirroring what cooks achieved by roasting, grilling, or searing. Over time, this compound’s profile made it indispensable for mimicking fresh-ground nuts, cocoa, and bakers’ crust in products ranging from instant soup to breakfast cereal. The chemical’s history reflects a push to replicate, safely and reliably, the tastes we expect year-round.
2,3-Diethyl pyrazine ranks high among specialty ingredients that turn bland processed foods into something consumers want to eat. In my experience with food technologists, this molecule usually comes in a colorless or pale yellow liquid, easy to handle and blend into dry or liquid bases. Manufacturers see it as a practical way to boost flavor without resorting to complex natural extracts. For those keeping track, this compound presses its advantage in foods needing a strong, quick-blooming aroma—think snack bars, nut-flavored cereals, or shelf-stable pastries. Its strength means only tiny amounts bring big results, offering a cost-effective option where natural extracts fall short or bring additional allergens.
A quick scan of the material safety data sheet shows 2,3-diethyl pyrazine boils at around 178-179°C, with a density just shy of water. It dissolves in ethanol and common organic solvents but not water. The scent stands out—roasted, nutty, a little sweet—a signature that’s hard to duplicate without creating distractions in the background aroma. In food production lines, its stable nature means you won’t see it degraded by gentle heating; it survives baking or extrusion far better than fragile natural volatiles. For the chemist, it’s neither acutely flammable nor dangerously reactive under normal processing conditions, which reduces worry during mixing and storage.
Every major supplier issues certificates of analysis detailing purity—often 97% or higher, with the rest mostly harmless byproducts like other pyrazines or trace solvents. The specific gravity, refractive index, and flash point usually show up on batch records. I noticed food safety laws push manufacturers to include “natural flavor” or “artificial flavor” depending on the origin and processing. For exporting products, the CAS Number 15707-23-0 becomes crucial for customs paperwork and tracking. In day-to-day work, clear, honest labeling keeps things transparent for downstream users who have to meet allergy labeling and food safety codes.
Most synthesis routes for 2,3-diethyl pyrazine start from ethyl-substituted precursors, with the process running through cyclization under mild conditions. Commonly, a condensation reaction between 2,3-butanedione and ethylenediamine under controlled temperatures delivers good yields. Labs optimized this by tweaking pH and solvent choices, moving away from old, less efficient methods using hydrazines or toxic reagents. Industrial-scale batches now use closed reactors to cut down on exposure and waste. Operators prize methods that cut byproducts, since purification can become the most expensive—and regulatory—headache.
Pyrazines readily accept substitutions, but the 2,3-diethyl version doesn’t react with common acids or bases at room temperature. More reactive environments, like high temperature or using strong oxidizers, might break the ring, but food manufacturers avoid these settings. Chemists sometimes look at further ethylation or try to attach oxygen-containing groups to tweak the aroma. Some recent papers describe catalytic oxidation processes that selectively introduce hydroxyl groups, which might improve its solubility in water-based systems. In practice, though, most companies stick with the core compound since derivatives rarely match the parent for both aroma and ease of regulatory approval.
2,3-Diethyl pyrazine pops up under several names, especially in global communications. Some catalogs call it “Pyrazine, 2,3-diethyl-”; others use EINECS 239-793-8 or the oh-so-memorable FEMA number 3179 for food flavoring registration. Older books refer to it with variations like ethylpyrazine or diethylpyrazine, but these days, precise IUPAC names win out. In consumer-facing food labels, direct naming rarely appears; instead, you see umbrella terms like “flavoring substances”—which hides the technical complexity behind simple ingredient lists.
In the flavor industry, safety takes more than a quick glance at toxicity tables. Handling standards draw on rules from groups like OSHA and the European Chemicals Agency. Gloves, goggles, and fume hoods reduce both skin and vapor exposure, though the compound’s moderate volatility means spills clear quickly with usual ventilation. The food-grade versions pass tough certification—no heavy metals above strict thresholds, solvent residues nearly undetectable. Batch records must document origin and traceability, as cross-contamination with allergens or industrial contaminants can trigger product recalls. Facilities set up regular audits and staff refreshers on safe handling, since even “generally recognized as safe” (GRAS) chemicals can present unexpected risks in concentrated form.
Look down the ingredient list of roasted nut snacks, creamy coffee syrups, or instant noodle flavor pouches and there’s a good chance 2,3-diethyl pyrazine hid behind the catch-all “flavor.” In my conversations with product developers, this compound often unlocks authentic profiles without relying on short-supply agricultural extracts. The reach covers baked goods, potato chips, dairy creams, plant-based meat analogues, and even pet foods. Some companies also tap it for specialty fragrances, especially those targeting earthy or gourmand notes. Its stability under heat and during long storage means it fits modern food processing needs, from extruded cereals to aseptically packaged soups.
Academic and industrial labs keep exploring better ways to synthesize, modify, and apply pyrazine derivatives. Patents show a steady trickle of innovations targeted at green chemistry—reducing solvents, using catalysts, and improving yields. Universities run sensory panels measuring how different concentrations influence not just base flavor but also aftertaste and aroma persistence. Teams in Asia, Europe, and North America also look at enzyme-mediated routes, hoping to qualify for “natural” labeling in cost-sensitive markets. Some work aims to extend the flavor’s application by making it more dispersible in aqueous solutions or pairing it with other aroma compounds to reduce off-notes.
Regulatory bodies take a hard look at every new or widely used food additive, and 2,3-diethyl pyrazine saw its fair share of safety studies. Early research flagged no acute toxicity at usage levels in foods and no clear evidence of bioaccumulation. Chronic exposure studies in rodents, done for GRAS status, pointed to high safety margins, but cumulative effects always demand long-term tracking in people. In real-world use, allergic reactions don’t seem common, but batch impurities or misuse could raise risk. The compound steers well clear of the spotlight compared with other synthetic flavorings linked to health scares, though continuous monitoring remains part of industry best practice.
As food and beverage companies shift toward cleaner labels and more sustainable production, the heat stands on synthetic pyrazines. Some research groups chase fermentation or biocatalytic production to better mimic “natural” pathways, aiming to sidestep consumer pushback against “artificial” tags. The tools of synthetic biology offer hope for renewable, biosourced alternatives, which could open up new regulatory doors and possibly lower costs. Demand grows for tailored flavor profiles in plant-based proteins, where 2,3-diethyl pyrazine’s roasted note masks off-flavors and makes alt-meats more palatable. The challenge ahead lies in answering both regulatory scrutiny and evolving consumer tastes, keeping up with clean, traceable, and sustainable production while maintaining the sensory punch this chemical delivers.
If you’ve ever wondered what gives roasted coffee beans their warm kick or what makes some cereal taste like it just came out of a bakery oven, a small compound called 2,3-diethyl pyrazine probably played a role. This chemical, though it sounds like something from a chemistry textbook, shows up in kitchens all over the world, even if you’ve never seen its name listed on a food label.
2,3-Diethyl pyrazine pops up mostly in the world of food flavoring. Food scientists use it for a very specific reason: it infuses foods with a distinct nutty, roasted, and bread-like note. Picture the aroma drifting from a fresh loaf of bread, or that inviting scent when you tear open a bag of toasted snacks. These bold flavors don’t just come from the oven; they also come from a careful mixture of ingredients, with 2,3-diethyl pyrazine sitting quietly among them.
Over the years, as more folks started craving real flavors and less of the “fake” stuff, flavor experts paid close attention to compounds like this one. Their goal? To build back real tastes, especially after processing can dull the punch of foods. Adding something like 2,3-diethyl pyrazine doesn’t just patch up these flavors. It revives that powerful bite we seek in smoked nuts, popcorn, or even energy bars.
I grew up with parents who always favored home-cooked meals. Still, foods outside our home seemed to deliver a richness that home kitchens sometimes miss, especially in terms of aroma. The reason behind this? Often, it was the work of flavor enhancers developed in labs. Synthetic flavors, despite getting some flak, make it possible to enjoy consistent tastes, especially at big scales. With crops and natural ingredients, seasons can change the flavor. A drop of 2,3-diethyl pyrazine eases that problem, keeping snacks or cereals tasting the same, season after season.
Those who aim for clean labels want to see more plant-derived or “natural” listings, but safe synthetics actually help keep products affordable. Real vanilla or almond flavors come with huge price tags. Compounds like this pyrazine do some heavy lifting, standing in when natural sources aren’t up to the task.
The big question for many shoppers is, “Is it safe?” No one wants to worry about what goes in their food. Regulatory agencies in the US, Europe, and Asia have already gone through mountains of safety studies on this compound. Their work ensures that the amount used in food sits safely below any level that might cause harm. Still, reluctance about synthetic chemicals isn’t likely to disappear soon. Many shoppers don’t like the sound of anything ending in “-zine” or “-pyrazine.”
Here’s the rub: The science says one thing, but trust takes time to build. Every year, I talk to folks who swear off packaged foods, convinced everything lab-made is dangerous. To shift those opinions, producers and regulators do best by showing transparency. Listing ingredients, describing their role, and being open about sourcing all help ease concerns.
If the food industry wants to keep using smart flavor-building tools like 2,3-diethyl pyrazine, then communication matters. People feel reassured when manufacturers and scientists talk straight—no jargon, no fine print. Working with culinary experts who bridge science and kitchen wisdom helps too. Flavor won’t vanish from shelves, but how companies talk about it can make all the difference.
Walk into a bakery as a batch of fresh bread hits the racks, and you might not know it, but you’re getting a whiff of chemistry at work. 2,3-Diethyl Pyrazine plays a big role in the world of aromas many people associate with home, warmth, or the comfort of a savory snack. This compound gives off a strong roasted, nutty vibe—think toasted seeds, or the hearty crust on a loaf of sourdough. I’ve worked on plenty of product tests in food labs, and one thing’s always clear: just a pinch of 2,3-Diethyl Pyrazine can change the whole atmosphere, especially in snacks and baked goods.
Anyone who ever opened a bag of roasted nuts or poured out a breakfast cereal probably ran into 2,3-Diethyl Pyrazine. This chemical doesn’t hide. Its notes come off as deeply toasted, almost reminiscent of peanut skins, warm popcorn, or roasted coffee beans fresh from the grinder. It’s not shy—there’s intensity there that brings depth and authenticity to flavor recreations. Think about the crackling skin on roast chicken, or the first sniff from a fresh jar of peanut butter. Those experiences share a piece of their punch with this molecule.
In my years working around food development, especially with plant-based alt-proteins, nailing the right roasted or meaty flavor shifted opinions from “nice try” to “wait, is this real?” For lots of us, the tiny details in taste make or break the experience. Synthetic flavors that miss that rounded, nutty edge always taste a bit off; they fall flat in comparison to counterparts finished off with a drop of this pyrazine. That nutty and earthy note bridges the gap between bland and memorable.
2,3-Diethyl Pyrazine belongs to the family that gives many browned or grilled foods their mouthwatering savor. This isn’t an accident. The Maillard reaction—the browning effect that makes roasted meats, breads, and vegetables taste incredible—often creates small amounts of pyrazines. But capturing and concentrating that particular ethereal aroma allows for new applications in the world of flavors, letting food scientists punch up the richness of everything from vegan cheese to energy bars.
Getting that ideal roasted profile proves tricky, though. Adding too much strips away pleasantness, giving food or beverages a type of burnt overtone that can turn stomachs. Just ask anyone who’s gone too heavy on the flavoring in a trial batch. Balance is key, and achieving it calls for careful blending with softer, sweeter background notes. I’ve had better luck mixing in mellow caramel flavors or creamy elements to keep the edge in check without drowning out the classic savory punch.
To improve results, it helps to look beyond just cranking up intensity. Start by working with small concentrations—incremental additions, constant tasting, and an understanding of how heat affects perception. Training young food technologists in practical sensory analysis, not just lab measurements, would move the industry closer to stellar outcomes. Approaching it from both a technical and a culinary bent means those roasted, nutty hallmarks of 2,3-Diethyl Pyrazine can become an asset, not a liability.
Familiar, homey, unmistakable: the right touch of this compound lifts comfort food to a new level, making every bite or sniff more enjoyable.
You pop open a bag of chips, catch a hint of roasted, nutty aroma—that might come from a flavor compound like 2,3-Diethyl Pyrazine. It’s got a knack for making things taste a bit more savory, with notes you’d find in toasted nuts or roasted grains. Flavor makers use it to boost the sensory punch of all sorts of snacks and baked goods.
I remember hearing arguments both ways about synthetic flavors. On one hand, these molecules help food companies add complexity to processed products, without always depending on pricier ingredients. The question about safety, though, sticks. 2,3-Diethyl Pyrazine isn’t just some random chemical dreamed up in a lab—it exists in tiny amounts in roasted coffee and some nuts. The version in your chips is synthesized, but it’s chemically identical.
Food safety rules don’t just roll out on the honor system. Agencies like the U.S. Food and Drug Administration (FDA) pay close attention to what goes into processed foods. The Flavor and Extract Manufacturers Association (FEMA) sticks this compound on its Generally Recognized As Safe (GRAS) list, which isn’t given out lightly. Toxicologists check how the body breaks it down, whether it builds up, and compare expected consumption to levels shown to cause harm in animal tests. So far, the available evidence suggests no nasty surprises at typical exposure levels.
Here’s the thing: lots of things in high doses cause problems. Even water. Animal tests show that 2,3-Diethyl Pyrazine doesn’t seem to cause cancer or birth defects at food flavoring doses. Most people consume only milligram amounts per day, far below the thresholds used in safety studies. Research from regulators in the U.S. and Europe keeps circling back to the same message—this molecule doesn’t cause trouble at the concentrations found in food.
Folks just want straight answers: can I trust what’s in my food? That’s a fair demand. Food safety isn’t just about FDA checks or industry rules; it’s also about public trust. Synthetic flavorings always set off questions, especially for people who want to stick to recognizable ingredients. Some shoppers get itchy about “artificial” anything, even if the molecule itself matches what’s in roasted peanuts.
Regulatory bodies have work to do. Constant review keeps things honest—they owe it to regular people to keep checking new data. If any study raises red flags, adjust the rules fast. Over time, some folks push for more frequent, independent updates instead of industry-driven ones. Labels could also work harder, moving away from fuzzy terms like “natural flavors” and listing out what’s in the food. Clearer information builds trust, even if the science says a substance causes no harm.
In my own kitchen, I reach for whole ingredients, but I’ve spent enough afternoons munching on factory-made snacks to know I’m no purist. Safety, for me, always comes down to how much, how often, and how honestly something is labeled. With 2,3-Diethyl Pyrazine, decades of research put the odds in the consumer’s corner, though open eyes and updated rules keep everyone safer down the road.
Any chemical professional who's worked with aromatic compounds knows a sloppy storage procedure can spark massive headaches—both on regulatory paperwork and in the actual lab. 2,3-Diethyl Pyrazine, with its strong nutty aroma, doesn’t take much before someone in the next room asks what you’re cooking. Like other pyrazines, it brings more than just a distinctive scent; it reacts with air, light, and heat in ways you don’t want to discover by accident.
A shelf in direct sunlight with the window open seems the fastest way to turn an expensive bottle into useless liquid. Instead, storage means picking a spot away from any heat source. Most labs rely on cool, dry cabinets—usually something in the 2°C to 8°C temperature range—for sensitive aromatics. Air exposure shortens shelf life, so screw caps or tight seals are essential. Silica gel packs get tossed in for humidity control.
2,3-Diethyl Pyrazine doesn’t ask for cryogenic freezers, but it does better in the refrigerator than on an open bench. Handling the bottle, I stick with gloves—less to avoid direct toxicity than to keep the residue off my skin and away from lunch breaks. Pyrazines love to linger.
Ignoring proper storage means risking changes in both scent and structure. 2,3-Diethyl Pyrazine isn’t the most reactive cousin in the chemical family, but left uncapped, it will soak up moisture from the air and pick up off-odors fast. The compound oxidizes when exposed to oxygen for too long. In my early grad school days, a forgotten pyrazine sample meant several weeks’ worth of failed runs on the chromatograph, all thanks to a compound that had turned funky. I learned to trust strict storage guidelines the hard way.
Volatile chemicals and poor ventilation create bigger problems. A few milliliters leaking or evaporating can fill a room with odor that even air scrubbers struggle to chase away. Some pyrazine derivatives irritate skin or eyes. A tightly closed container, preferably made out of amber glass, controls both risks.
Product data sheets from major suppliers like Sigma-Aldrich and TCI point to cool, dry, and well-ventilated storage spaces for most pyrazines, including the 2,3-diethyl variant. Research shows aromatic pyrazines maintain their structural stability and sensory properties longest when kept cold and protected from direct light. Glass, rather than plastic, wins out for long-term storage thanks to reduced permeability and no risk of plasticizer contamination.
If I were setting up a new lab, I’d order a set of small-capacity amber bottles and a dedicated refrigerator, secured away from food and drink. Labels should name the compound, concentration, and opening date. Training new researchers about the quirks of volatile organic storage heads off future problems. A simple logbook, where everyone notes what goes in and out of storage, prevents mystery spills and missing bottles.
Investing in ventilation and spill kits offers extra peace of mind. For household or small-scale use, a locked, childproof cabinet in a garage can work, so long as the bottle isn’t left to bake in summer heat.
Practical storage methods for 2,3-Diethyl Pyrazine grow out of both official guidelines and the kind of mistakes you only make once. Tight closure, low temperatures, and moisture control aren’t just good science—they’re what keep surprises out of the lab and the compound out of the emergency log.
The chemical industry has a knack for using words that sound almost cryptic. Take 2,3-Diethyl Pyrazine. On paper, it’s called C8H12N2 and its molecular weight sits at 136.2 g/mol. Short and sweet—yet these numbers only skim the surface of why this compound draws attention. Pyrazines show up quite a bit in nature, especially as flavor ingredients in roasted and grilled foods. Tossing a steak on the grill or biting into toasted bread, chances are your nose picks up some version of this structure.
My introduction to pyrazines didn’t happen in a lab but during a stint at a coffeehouse. Roasting a fresh batch of beans, the air thickens with nutty, earthy smells. That’s not magic—it’s molecules such as 2,3-Diethyl Pyrazine transforming a bunch of flat beans into an aroma-packed brew. Food companies bank on this. Even the faintest bit of 2,3-Diethyl Pyrazine can deepen flavors or round out a synthetic food blend. Consumers rarely imagine food scientists hunched over a bench manipulating formulas, but these tweaks go a long way.
Some folks worry any chemical with a multisyllabic name spells trouble. Regulatory bodies, including the FDA, keep pretty close tabs on compounds like this. According to the FEMA GRAS (Generally Recognized as Safe) list, 2,3-Diethyl Pyrazine clears the bar for use in traces as a flavoring agent. Food safety isn’t just about hygiene practices; it’s about routine checks and clear labeling, especially since allergies and sensitivities can make even the smallest dose a health risk for some.
Outside the pantry, this compound offers lessons in how everyday life and science bump into each other. Synthetically produced 2,3-Diethyl Pyrazine helps ensure flavor consistency between batches of chocolate or roasted nuts. Factories can’t always count on agricultural production to deliver the exact taste year after year, which means even luxury brands use a dash of chemistry to protect their signature flavor. Some might see this as “cheating,” but anyone who’s tasted a flavorless tomato in winter knows that fresh isn’t always best. Smart chemistry can help make up the difference.
One thing many ignore—food chemistry doesn't always solve every problem. Some synthetic flavorings can leave products with a strange aftertaste or cause reactions in sensitive people. Moreover, overreliance on artificial flavoring might reduce demand for traditional farming, affecting jobs and local economies. To avoid these pitfalls, food producers should combine science with natural sourcing wherever possible. Consumer choice matters here. Pushing for more detailed labeling makes room for people to pick what aligns with their preferences and health.
At the end of the day, a chemical formula like C8H12N2 might look sterile, but trace it back to what lands on the plate and its story turns practical. Everyone from home cooks to commercial food manufacturers stands to benefit from a clearer look at what's behind each flavor. By giving shoppers solid information and making safe, balanced choices, both science and tradition can share a spot at the table.
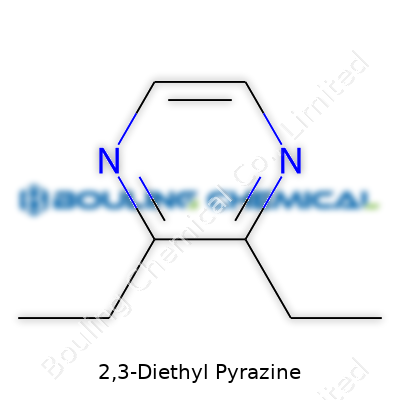
| Names | |
| Preferred IUPAC name | 2,3-Diethylpyrazine |
| Other names |
2,3-Diethylpyrazine Pyrazine, 2,3-diethyl- Diethylpyrazine |
| Pronunciation | /tuː θriː daɪˈɛθɪl paɪˈræziːn/ |
| Identifiers | |
| CAS Number | [15707-23-0] |
| Beilstein Reference | 1205603 |
| ChEBI | CHEBI:134564 |
| ChEMBL | CHEMBL511058 |
| ChemSpider | 21569755 |
| DrugBank | DB08437 |
| ECHA InfoCard | 19-209-010-1 |
| EC Number | 216-041-6 |
| Gmelin Reference | 7796 |
| KEGG | C16235 |
| MeSH | D017967 |
| PubChem CID | 13602 |
| RTECS number | UG8400000 |
| UNII | QE7R42DV3Z |
| UN number | NA1993 |
| CompTox Dashboard (EPA) | DTXSID8032068 |
| Properties | |
| Chemical formula | C8H12N2 |
| Appearance | Colorless to pale yellow liquid |
| Odor | nutty; roasted; earthy |
| Density | 0.965 g/cm3 |
| Solubility in water | Insoluble |
| log P | 1.61 |
| Vapor pressure | 0.0185 mmHg at 25°C |
| Acidity (pKa) | 15.14 |
| Basicity (pKb) | 1.64 |
| Magnetic susceptibility (χ) | -61.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.4990 |
| Viscosity | 1.029 mPa·s (25 °C) |
| Dipole moment | 1.61 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 269.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -4162.7 kJ/mol |
| Hazards | |
| GHS labelling | GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. |
| Precautionary statements | Precautionary statements of 2,3-Diethyl Pyrazine: "P264, P270, P305+P351+P338, P337+P313 |
| Flash point | 99 °C |
| Autoignition temperature | 482 °C |
| Lethal dose or concentration | LD50 (oral, rat): 4600 mg/kg |
| LD50 (median dose) | LD50 (median dose): 600 mg/kg (rat, oral) |
| NIOSH | QW1353000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 5 mg/m³ |
| Related compounds | |
| Related compounds |
2,5-Diethylpyrazine 2,6-Diethylpyrazine 2,3-Dimethylpyrazine 2-Ethyl-3-methylpyrazine 2,3,5-Trimethylpyrazine |