Stories behind food chemistry often echo the history of taste itself. Pyrazines, those small molecules behind so much of what smells and tastes good, found fame after flavor chemists dug into the structure of roasted and cooked foods back in the 1960s and 1970s. The work tracked the warm, nutty notes in everything from coffee to baked bread, and 2,3-Diethyl-5-Methyl Pyrazine quickly became one of the stars among its relatives. Food scientists, driven by the quest to reproduce roasted and nutty sensory qualities, refined their methods to make and analyze this molecule, setting the foundation for decades of research and development in both natural and artificial flavorings.
2,3-Diethyl-5-Methyl Pyrazine goes way beyond textbook chemistry. This compound’s signature aroma lands near toasted nuts, popcorn, cooked grain, and savory snacks. Even in tiny doses, its strong, pleasant flavor profile carries through, which is why food scientists and perfumers keep coming back to it. Industrial producers usually offer it in pure and highly concentrated forms, and smaller formulation companies often blend it in customized mixtures, making it essential for imitating or enhancing roasted foods and snack seasonings.
It shows up as a slightly oily liquid under most laboratory conditions—pale yellow, with a boiling point in the neighborhood of 179-184°C at atmospheric pressure. The molecular formula, C9H14N2, renders it slightly heavier than water but not by much. It dissolves in ethanol and ether yet doesn’t play well in water. Its optical properties stay pretty plain, making it easy to pick out and analyze using standard GC techniques. The magic, of course, lies in its volatility; this compound moves through the air at room temperature, carrying its flavor into everything from packaged snacks to freshly roasted coffee.
Companies making or distributing this molecule must get down to tight technical numbers. Purity generally runs above 98%, as off-flavors stand out even at low concentrations. Trace solvents, usually leftover from synthesis, must stay under strict limits. Makers include information on the label not only about content percentage, but batch number, recommended storage temperature, and shelf life. Transparency matters, so full compliance with regulations from authorities like the FDA in the USA or EFSA in Europe stands as the rule, not the exception. Many companies offer safety data sheets, which flag hazards and give clear instructions on what to do in case of accidental spill or ingestion.
Synthesizing 2,3-Diethyl-5-Methyl Pyrazine can be done in a few distinct pathways, though most rely on condensation reactions involving the right substituted 1,2-diketones and ethylamines. Early on, bench chemists ran these reactions in glassware with careful temperature control, but commercial-scale preparations now rely on stainless-steel reactors, often run under nitrogen and equipped for rapid cooling to prevent unwanted byproducts. Purification uses fractional distillation and chromatographic separation to remove traces of related compounds and trapped reagents, since the human nose picks out these slip-ups even in parts per billion. The entire production process walks a tightrope between yield, efficiency, and preserving that all-important aroma profile.
The pyrazine nucleus stands up to quite a bit of chemical tinkering. Minor tweaks—adding or swapping alkyl groups, for example—alter the sensory impression, moving a flavor from roasted peanut to popcorn to cereal. That’s key for flavorists working to replicate not only the smell of a single ingredient, but the balance in something more complex like a trail mix or breakfast bar. 2,3-Diethyl-5-Methyl Pyrazine also takes part in controlled oxidation reactions, which produce even more nuanced sub-flavors, and its core structure serves as a building block for newer classes of flavorings that find use in both food and personal care items.
Anyone reading a flavor label may spot this molecule under alternative names. It pops up as 2,3-Diethyl-5-methylpyrazine, Ethyl Methyl Pyrazine, and sometimes as part of broader “alkyl pyrazine” blends. Food additive numbers change by region, so EU product sheets usually reference the FEMA GRAS number or FLAVIS system, giving importers and manufacturers a clear route through customs and compliance. Brands in the flavor market sometimes assign catchy trade names, but the core identifiers trace back to the molecular structure.
Extended exposure to concentrated pyrazines can put workers at risk for skin or mucous membrane irritation, so outfits handling large volumes rely on protective gear, fume hoods, and well-trained staff. Regulations shape almost every move, from how companies document workplace exposure to their storage protocols. Strict adherence to local fire codes and chemical handling procedures reduces incidents in warehouses and labs. Most labels carry hazard pictograms with clear first aid instructions; probably not many people realize that, for all their culinary cachet, pyrazines require careful handling behind the scenes.
This compound goes far beyond food, though its main home sits with flavor houses and processed snack makers. It amplifies natural nut and grain notes, boosts roasted flavors in everything from breakfast cereals to plant-based meats, and even lands in pet foods designed to appeal to both human buyers and animal appetites. Some tobacco blends, candles, and specialty cleaning products feature it in trace amounts to mimic the smell of warm bread or roasted beans. Beverage companies use it up to regulatory limits in specialty drinks, sometimes to give malt or brown notes to non-alcoholic alternatives. The molecule, in the right hands, offers an ace up the sleeve for anyone shaping taste or smell.
Academic labs and industry groups walk hand in hand looking for smarter production methods that sidestep waste and reduce the need for harsh reagents. Biotechnologists hope to engineer yeast or bacterial strains that ferment their way to 2,3-Diethyl-5-Methyl Pyrazine using renewable feedstocks. Flavor scientists chase better ways to mimic the molecule’s impact on the brain’s sensory pathways, linking breakthroughs here to advances in food formulation and novel consumer experiences. Data from gas chromatography-olfactometry studies inform how the molecule interacts with everything from fats to plant proteins, unlocking new recipes and more sustainable production cycles.
Nearly every study lines up to confirm that pyrazines at food-grade doses stay well below known toxicity thresholds, with the structure of 2,3-Diethyl-5-Methyl Pyrazine placing it in the “generally recognized as safe” category. That doesn’t make it a free-for-all: regulatory bodies keep a close watch on cumulative exposures, oxidation products, and interactions with other ingredients. Chronic exposure in lab animals, usually at concentrations higher than any regular diet would deliver, sometimes triggers mild changes in metabolic markers, pushing researchers and regulators to keep reviewing available toxicological data as new forms and mixtures appear. The challenge for safety experts remains keeping up as more sectors look to these molecules for new applications.
The path ahead for 2,3-Diethyl-5-Methyl Pyrazine runs through both familiar territory and uncharted ground. In food, advances in plant-based protein and dairy substitutes push flavorists to keep innovating ways to mask undesirable notes and reinforce the comforting flavors of toasted grains and nuts. Regulatory standards may shift as new research shines light on long-term exposure profiles, which means flavor houses and manufacturers need to stay agile and proactive. Developments in green chemistry may deliver more pathways to synthesize it, cutting down the chemical waste and energy costs standard in today’s industry. With interest in authentic sensory experiences only growing, this small molecule promises to keep making waves in everything from next-generation snacks to clean-label food technology.
Walking through a snack aisle, the smell of roasted nuts and chips can grab your senses before your bag even opens. A big player behind that inviting aroma is 2,3-Diethyl-5-Methyl Pyrazine. People outside food science circles might not ever come across the name, but if you love freshly roasted peanuts or crunchy cornflakes, you’re already a fan.
Pyrazines are champions in the world of flavor creation. Manufacturers count on 2,3-Diethyl-5-Methyl Pyrazine because its scent brings out the roasted, nutty notes that define crispy snacks. It carries the kind of flavor that transforms plain rice cakes or breakfast cereal into something you crave. Food tastes bland without the deep, toasted notes you get from the right pyrazine.
Look at food manufacturing today: flashy packaging helps, but if a chip doesn’t actually taste like a chip, the product slips off grocery lists. A handful of companies have carved out fortunes simply by mastering the science of flavor, and 2,3-Diethyl-5-Methyl Pyrazine is part of that winning formula. I remember biting into a new “honey nut” breakfast cereal as a kid and thinking it tasted like real roasted peanuts, not candy. Years later, I learned this compound played a starring role.
It even pops up in gourmet coffee. Roasters use pyrazines to mimic the caramelized, nutty profiles that coffee lovers seek out. Some fine chocolates owe their deep flavor layers to traces of this same compound. In savory foods, manufacturers blend it into seasonings to boost that “just-cooked” bite. If you’ve ever eaten instant noodles that smelled like something straight from a street kitchen, chances are, a pyrazine like this helped.
Synthetic flavor chemistry isn’t about faking food. It’s often about making the real thing affordable, consistent, and safe from crop failures or wild price swings. Spiking the flavor of cereals, snacks, and even pet food with a compound like 2,3-Diethyl-5-Methyl Pyrazine can keep production costs steady. More predictability here beats the headache of waiting for a peanut harvest to come in every year.
Some critics worry that this sort of chemistry hides what’s actually in our food. Labels often say “natural flavors,” but they rarely spell out what goes into the mix. In one sense, the science can feel secretive, but the alternative leaves us with food that spoils fast or fails to trigger any real sense of enjoyment. People deserve clear information, and agencies like the FDA keep tabs on food additives like this pyrazine. It’s safe at the tiny levels used in snacks and cereals, but independence in regulatory oversight should keep pace as innovations keep rolling out.
Pressure is on flavor houses to keep ingredients as natural as possible, or at least to show where each part comes from. Plant-based and clean label trends aren’t fading. Companies now look to create “bioidentical” flavors by fermenting bacteria rather than cooking chemicals in a lab. Pyrazines, including 2,3-Diethyl-5-Methyl Pyrazine, are part of that broader shift. Some forward-thinking groups are asking if flavor solutions can come from food waste streams, like spent grains, which already contain the aroma profiles people want.
True flavor isn’t about tricking taste buds. It’s about making basic food taste so much better. Without compounds like 2,3-Diethyl-5-Methyl Pyrazine, a lot of our favorite foods would disappear from shelves or fade into blandness. All the marketing in the world can’t fix food that people don’t actually crave.
Plenty of folks glazed over in high school chemistry. The jumble of numbers and words in names like 2,3-Diethyl-5-Methyl Pyrazine scared off even the most curious. Yet anyone who’s spent time in a kitchen has likely brushed up against this molecule’s character, at least in spirit. Pyrazines tend to deliver the punch behind roasted, toasted, and nutty flavors, even if their chemical name never hits a menu.
I’ve worked with enough roasted peanuts, coffee beans, and dark bread crusts to spot the fingerprints of a pyrazine right away. Bring a nose close to freshly ground coffee and you sniff a dry, warm scent that lingers at the back of the palate. 2,3-Diethyl-5-Methyl Pyrazine sharpens that aroma, rooting deep in earthy territory, while giving off an almost woody, nutty bite.
Aromatically, it leans toward toasted hazelnut and peanut skins—a flavor that’s hard to capture without real fire or heat. It skips past generic “nutty” and lands on the specific: the moment a peanut shell cracks, the sort of flavor memory that sticks from county fair snacks and homemade nut brittle. There’s edge in this molecule: a clean, sharp, green bell pepper note sits beneath it, likely from its pyrazine backbone—similar to what you get in raw, sliced bell pepper, but dialed down, more refined, less grassy. Some chemists mention the “earthy” and “musty” qualities, and that tracks. The world’s best truffles share molecules with this family, which goes a way toward explaining why a trace of the stuff can turn a bland nut spread or low-grade chocolate into something richer.
Food scientists keep pyrazines handy when blending flavors for snacks, coffee, or roasted nuts, mostly because these compounds fill in the blanks left by cheaper raw materials. I’ve tasted enough off-brand peanut butters and “roasted” chips to know that authentic flavor rarely comes cheap, but a nudge of the right pyrazine creates the illusion of higher quality roasting. In that way, it fools the senses just enough to suggest a longer, deeper roast or a fresher grind than was ever actually the case.
Adding a precise drop of 2,3-Diethyl-5-Methyl Pyrazine to a recipe brightens the overall profile when something feels flat or muted. This isn’t about trickery; it’s about rounding out flavors so they match the expectations of smell and taste. Companies use it to bring back lost character in food that’s been processed, stored, or shipped across continents. If you’ve ever wondered why that convenience store snack actually reminds you of a fancy holiday nut tray, you’re probably tasting a pyrazine at work.
There’s an art to not letting this compound overpower a blend. Pyrazines pack such strength that even a trace amount sings out. One heavy hand, and the snack tastes scorched; too light, and the depth vanishes. I’ve learned to respect that fine line, the same way a chef is careful with strong spices like cumin or smoked paprika.
Food companies with an ear to the ground keep a tight watch for consumer feedback about “burnt,” “musty,” or “chemical” notes, often tracing complaints back to tweaks in the pyrazine balance. Getting it right means products that taste fresher, richer, more comforting—the kind people come back for without quite knowing why.
Browsing the labels on snacks and perfumes has turned into a chemistry quiz these days. The name 2,3-Diethyl-5-Methyl Pyrazine tends to stand out, especially if you care about what goes into your food or the bottle you spray on your wrist. My earliest encounters with these sorts of tongue-twisting names usually left me scratching my head, wondering what role all these things play — and if they’re actually safe.
If you’re not familiar, this compound falls into the category of pyrazines, a group known for their strong nutty, roasted, and earthy aromas. Cheddar chips, roasted nuts, and some plant-based meats rely on flavors like this to make people crave just one more bite. In perfumery, a molecule like this brings a fresh, almost edible warmth to certain blends, the sort that lingers fondly on your jacket after a busy afternoon.
The U.S. Food and Drug Administration recognizes many pyrazines as safe for food use, including several similar in structure to 2,3-Diethyl-5-Methyl Pyrazine. The compound itself is listed by international safety panels, such as the Joint FAO/WHO Expert Committee on Food Additives, as a flavoring agent that does not raise big red flags in the amounts people actually eat.
Safety boils down to both the amounts used and the context. In food, levels run extremely low, measured in parts per million. Those who make the rules set exposure guidelines far below the threshold where research has shown any biological effects. Animal studies don’t link it to cancer, reproductive problems, or organ damage at realistic exposure levels. Chronic overexposure hasn’t popped up as a concern, and the European Flavour Association also puts it in the safe-for-flavor-use column.
Fragrances come with their own set of rules. Perfume formulas operate under industry-wide safety codes, updated each few years after toxicologists check new and existing research. For something like this pyrazine, the story’s much the same: it appears at such low concentrations in most products that human health risks stay low.
That doesn’t mean there’s nothing to watch. Bodies react to scents and flavors in all sorts of ways. Some folks have noses that catch even the faintest synthetic aroma and turn it into a mighty headache or make a mild rash pop up. It helps to remember that just about any aroma or flavor, synthetic or natural, can do that — Allergies don’t discriminate. I’ve met people who can’t walk past a field of wildflowers without sneezing, and others who skip certain snacks because one whiff triggers a reaction.
Environmental effects spike new questions about these chemicals. Pyrazines break down fairly quickly in air and water, much quicker than heavier, less-soluble molecules. We’ve learned to look out for cumulative effects that aren’t obvious at first glance, especially in waterways, but these ingredients aren’t showing up as major pollutants. Whether that will stay true as the number of flavor molecules grows is another question entirely.
The best safeguard comes from clear rules and honest labeling. Regulators need to keep demanding up-to-date toxicology data on food and fragrance ingredients. At the same time, it makes sense for brands to give people better information about their products, using plain language instead of hiding behind vague chemical codes. If someone wants to avoid a particular flavor or scent, that decision should be made easy — not locked behind industry jargon or fine print.
It’s worth remembering that safety isn’t a static picture. Science moves fast, uncovering new risks or unexpected benefits as different molecules turn up in hundreds of foods and fragrances. For 2,3-Diethyl-5-Methyl Pyrazine, the weight of current evidence places it in the low-concern group, but that should never be an excuse to stop asking questions or demanding transparency from those who put these compounds into things we eat and breathe.
2,3-Diethyl-5-Methyl Pyrazine doesn’t get the same attention as big-name food additives, yet you catch it any time you bite into a bowl of rice or crunch a cracker with a “baked” note. This molecule shows up in the world’s flavor industry because it nails down that savory, nutty aroma, one that instantly reminds people of comfort foods and homestyle cooking. Its effect is bold, even at the lowest levels. That great punch lets creators stretch supply far, and it saves companies a lot of money while keeping blends from getting too heavy-handed.
Anyone who’s tried tinkering with flavors knows these sorts of compounds can become overwhelming fast. You can’t just sprinkle in 2,3-Diethyl-5-Methyl Pyrazine by eye. Food scientists agree: a little goes a long way. Typical concentrations settle in the range of 0.01 to 1 parts per million (ppm) in finished food. That means for a ton of product, you barely add a few grams. Often, you might see dosing levels at the lower end—0.05 to 0.2 ppm does the trick for most nutty or roasted snacks. That level keeps the taste authentic without overpowering everything else in the recipe.
Working in a lab years ago, I spent days tweaking these levels. Nobody wanted a pretzel that smelled like burnt peanuts or a rice cake that stung the nose. It turns out, overshooting even by a tenth of a ppm could throw off an entire production run. In the factory, operators measured out flavors with high-precision scales, not cups or scoops. That’s true respect for the molecule’s firepower. Food safety groups back this caution, since the line between tasty and weird sits much closer together with strong pyrazines.
Food laws don’t give blanket permission to just dump pyrazines into anything. Agencies like the FDA and European Food Safety Authority have their eyes on these aromatic chemicals. Right now, 2,3-Diethyl-5-Methyl Pyrazine lands on the GRAS (Generally Recognized As Safe) list for use in food—with careful dosing. The Joint FAO/WHO Expert Committee on Food Additives recommends maximum levels similar to industry practice: sticking to the lowest edge that does the work.
This checks out in real kitchens. Any chef or product developer paying attention looks at the label and does the math. Most suppliers also demand clear documentation showing proper use—not only for safety, but also because flavor houses hate recalls and bad headlines.
It’s tempting to chase “more” when a flavor wins over tasters. Yet, real skill shows up in restraint. People sometimes ask if there’s a shortcut—if lower-quality base ingredients can be masked with a heavy dose. Turns out, big-time overuse of 2,3-Diethyl-5-Methyl Pyrazine just points a spotlight on off-notes and processing flaws. You get a snack that tastes weirdly artificial and nobody comes back for seconds.
So, reaching for pure pyrazine might work for a flavor lab, but for anyone cooking in volume or selling to a real audience, keeping levels low—following 0.01 to 1 ppm—works best. Training staff, labeling things correctly, and setting up checks on each batch offer a safety net. Most producers keep master blends ready at the right dosage so mistakes drop off. That mix of careful measurement and sensory testing brings out the best in these powerful flavor agents, keeping products tasty and safe for everyone at the table.
A chemical like 2,3-Diethyl-5-Methyl Pyrazine means more than lab talk to me. I’ve shared space with enough glassware and flavors to know that shelf life rarely becomes clear until you mishandle a batch, lose money, or expose coworkers to something risky. Some would say a simple molecule won’t cause much fuss, especially one used for scents and flavors, but the everyday story tells a deeper tale. Mistakes with storage can cut the value of an expensive flavor chemical to zero, and if the pyrazine spoils quietly, the final product changes and nobody can explain why.
Most pyrazines, including 2,3-Diethyl-5-Methyl, have good staying power, but it isn’t unlimited. A typical manufacturer says two years if the original sealed container survives tamper-free. Still, that date fades fast if you ignore some basics. Think about light—exposure breaks down aroma chemicals. Oxygen? That’s the slow thief in the room, chipping away at quality every time you open the bottle. Humidity clings to everything too, creeping into cracks, and a little unseen moisture easily ruins sensitive organics. Temperature matters plenty; swings or outright warmth speed up every chemical reaction you wish would stop.
Several years back, a proud new flavor technician told us, “I just screw the cap tight, no big deal!” By the end of summer, nothing in his box tasted right. Simple habits matter most. Dry, cool, and dark should always come to mind—think of a wine cellar, only more cautious. I stick to refrigerators if I have a choice (4–8°C is ideal), and refuse to leave chemicals near heat sources like windows or hot pipes. Silica gel packets work wonders for pulling out sneaky moisture, and bottles with tight-sealing caps (not dropper or snap tops) keep air at bay.
I always check for odd color, chunks in what should be a liquid, or a hint of off-smell—any of those say stop using the batch. The best-run kitchens and labs never decant into unmarked bottles. The label always includes the opening date, which helps everyone remember not to use anything past its prime.
One change that always pays off: move away from large, slow-moving bottles. Smaller, clearly labeled portions keep exposure minimal and reduce the number of times air gets inside. For those running a busy lab or production space, think about an inventory system flagged by expiration. Regular audits save product and catch errors before they wreck a whole set of batches. It never hurts to keep a master list that links each lot number to the vendor, date received, and notes about storage conditions along the way.
Sticking to good storage keeps flavor and safety intact. As a bonus, it saves money—no one wants to throw away half a bottle because it sat too long in the wrong spot. Most accidents or mistakes could be traced back to lazy habits or wishful thinking. Pyrazines are just one chapter in a long story about chemical care. Handle them right, and you can trust every blend and formula that follows.
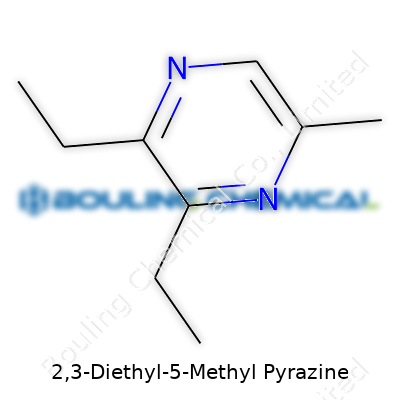
| Names | |
| Preferred IUPAC name | 2,3-diethyl-5-methylpyrazine |
| Other names |
2,3-Diethyl-5-methylpyrazine 5-Methyl-2,3-diethylpyrazine Pyrazine, 2,3-diethyl-5-methyl- 2,3-Diethyl-5-methyl-1,4-diazine |
| Pronunciation | /ˈtuː θriː daɪˈɛθɪl faɪv ˈmɛθɪl paɪˈræziːn/ |
| Identifiers | |
| CAS Number | [59160-78-6] |
| 3D model (JSmol) | `3Dmol.js?load=mol:data:chemical/x-mol;2,3-Diethyl-5-Methyl.Pyrazine` |
| Beilstein Reference | 100926 |
| ChEBI | CHEBI:142425 |
| ChEMBL | CHEMBL502960 |
| ChemSpider | 124162 |
| DrugBank | DB08206 |
| ECHA InfoCard | 03e63538-120f-45d2-ae2a-761e216f8855 |
| EC Number | 620-900-9 |
| Gmelin Reference | 166486 |
| KEGG | C10502 |
| MeSH | D017967 |
| PubChem CID | 12350 |
| RTECS number | UJ8245000 |
| UNII | 69U49F6R6O |
| UN number | NA |
| CompTox Dashboard (EPA) | 2RV8X397GB |
| Properties | |
| Chemical formula | C9H14N2 |
| Molar mass | 192.284 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | nutty, roasted, peanut, earthy |
| Density | 0.936 g/cm3 |
| Solubility in water | Insoluble |
| log P | 2.46 |
| Vapor pressure | 0.102 mmHg at 25°C |
| Acidity (pKa) | 14.2 |
| Basicity (pKb) | 2.83 |
| Magnetic susceptibility (χ) | -71.7×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.4910 |
| Viscosity | 1.295 mPa·s (25 °C) |
| Dipole moment | 1.76 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 389.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -4436.8 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. Toxic to aquatic life with long lasting effects. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | Hazard statements: "H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P273, P301+P312, P305+P351+P338, P337+P313 |
| Flash point | Flash point: 93 °C |
| Autoignition temperature | 470 °C (878 °F; 743 K) |
| LD50 (median dose) | LD50 (median dose): >2000 mg/kg (rat, oral) |
| NIOSH | SKD3QDJ306 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 2,3-Diethyl-5-Methyl Pyrazine is not specifically established by OSHA. |
| REL (Recommended) | 1 mg/m³ |
| IDLH (Immediate danger) | Not Listed |
| Related compounds | |
| Related compounds |
2,3-Dimethyl-5-ethylpyrazine 2,3,5-Trimethylpyrazine 2-Ethyl-3,5-dimethylpyrazine 2,3-Diethylpyrazine 2-Methyl-3-ethylpyrazine Pyrazine |