Chemistry didn’t always pay attention to minor heterocyclic systems like pyrazines. They stood in the shadow of bigger, splashier molecules through much of the early twentieth century. That changed as agrochemical and pharmaceutical industries ramped up their search for new scaffolds during the latter half of the century. By the 1970s, researchers had mapped out dozens of synthetic paths to halogenated pyrazines. 2,3-Dichloropyrazine emerged from this push for more versatile building blocks, attracting interest because its robust ring tolerated functional group modifications, crucial for drug design and crop protection research. Several patents from the 1980s document European and Japanese chemists competing to refine the process. This wasn’t blind academic curiosity—makers of fungicides and pharmaceutical intermediates began to incorporate 2,3-dichloropyrazine into their workflows, building a practical bridge from early bench-top exploration to full-scale production.
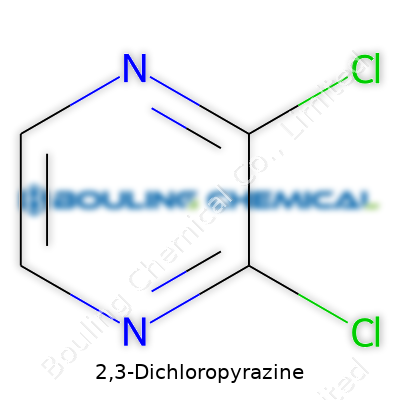
2,3-Dichloropyrazine stands as a solid intermediate bridging base chemicals and essential end-products for many industries. Its six-membered ring, seasoned with nitrogen at the 1- and 4-positions and chlorines at the 2- and 3-positions, offers more than just structural quirk. Chemists appreciate its tractability, seeing it as both a resilience model and a springboard for more complicated derivatives. Companies engaged in advanced materials or fine chemicals rarely overlook it. I've noticed research labs often keep a bottle on hand, since its versatility makes it valuable even on short notice—no wasted shelf space here. Prices fluctuate, but availability holds up thanks to steady, predictable demand among specialty chemical suppliers.
2,3-Dichloropyrazine usually turns up as a white to light-yellow crystalline solid. With a melting point hovering around 60–63°C and a boiling point above 200°C, it sits firmly in the thermal window that suits many transformations in organic synthesis. This substance barely dissolves in water, making it easier to handle and purify compared to more soluble analogues, though it finds better solubility in polar organic solvents like DMSO or acetonitrile. Stability under standard storage conditions speaks to its popularity in research and industrial settings. Chlorines rooted on the heterocyclic ring not only bump up its chemical resistance but set the stage for diverse nucleophilic substitution chemistry. A careful technician, properly masked and gloved, finds little trouble in measuring or transferring this crystal, whose faint, pungent aroma signals the world of chlorinated heterocycles that always demands respect for safety.
Reputable suppliers outline the purity of 2,3-dichloropyrazine—often between 97% and 99%—along with key measures such as melting point, water content by Karl Fischer titration, and GC/HPLC chromatographic analysis. Labels usually flag hazard statements about skin and eye irritation, inhalation risks, and flammability, ensuring even a moment’s lapse won’t put health at unnecessary risk. The chemical sits under UN and GHS classification as a hazardous substance, so containers typically feature bold pictograms, hazard numbers (like H315 for skin irritation), and clear batch identifiers. In my own work, careful checking of certificates of analysis avoided any hiccups, especially where trace impurities meant the difference between a dull and a vibrant product. Regulations may differ country by country, but comprehensive labeling remains a non-negotiable responsibility.
You don’t usually turn to one pot for 2,3-dichloropyrazine and get lucky—a multistep route offers more control over selectivity and yield. Most syntheses start with pyrazine as the backbone. Direct chlorination using reagents like phosphorus pentachloride or thionyl chloride can generate mixtures, but the most reliable method involves pre-functionalizing pyrazine or employing directed ortho-lithiation. Another path involves cyclization of dicarbonyl chlorides with hydrazine, followed by stepwise chlorination. The key challenge involves avoiding over-chlorination at positions 5 and 6, which requires temperature control and careful stoichiometry. These routes scale well, as evidenced by industrial patents designed for kilogram and ton-level runs.
The beauty of 2,3-dichloropyrazine lies in its willingness to swap out those chlorines for larger, more complex groups. Nucleophilic aromatic substitution comes easy, especially in the hands of a chemist with a good leaving group at hand—thiols, amines, and alkoxides all find themselves attached to the ring after mild heating. Some pharmaceutical applications depend on these modifications, opening the door to a raft of bioactive structures. Metal-catalyzed coupling, especially Suzuki-Miyaura and Buchwald-Hartwig reactions, permit further tweaking, bolstering its appeal in medicinal chemistry. During my time in drug discovery, this flexibility allowed our lead optimization teams to rapidly create libraries with small changes in lipophilicity and hydrogen bonding, unlocking new structure-activity relationships.
2,3-Dichloropyrazine doesn’t march under a single banner. Depending on the supplier or jurisdiction, you’ll see synonyms like 2,3-dichloro-1,4-diazine and dichloropyrazine. The CAS number 4857-20-3 provides a reliable anchor when names start to look too similar. Commercial catalogs may list the product as DCP, Pyrazine, 2,3-dichloro-, or just as its molecular formula, C4H2Cl2N2. Robust supply chains depend on this clarity and reduce confusion, making sure researchers and plant chemists talk about the same thing, regardless of local language or trade names.
Safety standards matter as soon as you start handling halogenated heterocycles. MSDS sheets spell out irritant properties and the need for gloves, goggles, and lab coats, but it goes deeper than checking boxes—proper ventilation, containment, and spill procedures stop small mishaps from turning into lost workdays. Any open reaction needs a working fume hood, and the solid should never be handled without a spill tray on the balance. Disposal aligns with chlorinated waste protocols, preventing harmful byproducts from leaching into water or soil. I’ve run QA audits where improper labeling or uncapped vials flagged a wider culture problem, so regular staff training pays off. Proper record-keeping, emergency eyewash stations, and periodic review of safety protocols keep operations smooth and in regulatory compliance.
Agriculture and pharmaceuticals draw most heavily on the strengths of 2,3-dichloropyrazine. Crop protection chemicals, especially new fungicides and herbicides, use it as a core scaffold for molecules designed to break through resistant organism defenses. Synthetic chemists also see potential in PET imaging agents and new anti-infective leads. Material scientists find utility in crafting dyes and corrosion inhibitors, especially where resistance to weathering and aggressive environmental factors counts. Research in my network points to growing interest in specialty polymers—crosslinkers involving the dichloro motif set up with remarkable tunability in the final product’s mechanical features.
Academics and R&D chemists approach 2,3-dichloropyrazine from two main angles: improving the synthetic route and expanding application. Greener chlorination protocols, lower-waste purification strategies, and catalyst innovations dominate industry journals, reflecting a broader drive across specialty chemicals to address sustainability. High-throughput screening of pyrazine derivatives shows fresh promise in anti-viral compounds and as starting points for kinase inhibitors. Multinational agrochemical companies continue to file patents each year, each chasing more effective and less persistent compounds for safer, next-generation crop solutions. My talks with bench chemists suggest that bottlenecks remain at scale-up and process safety, so solutions focus equally on yield and health/environmental impact minimization.
Toxicologists keep a watchful eye on chlorinated aromatics. 2,3-Dichloropyrazine doesn’t escape scrutiny—rat studies and in vitro data highlight potential for moderate skin and respiratory irritation but suggest comparatively low acute toxicity. Environmental chemists run soil and water assays, often reporting some persistence but manageable degradation over weeks. The structure rarely triggers strong mutagenicity or reproductive warnings, but regulatory agencies in Europe and North America still require finished formulations to undergo robust, multi-generational studies. In the lab, experiencing a minor splash reaffirms that even “manageable” risk means real discomfort if careless—these moments reinforce the real-world importance of safety protocols and careful chemical stewardship.
Chemists will keep turning to 2,3-dichloropyrazine as the search intensifies for new high-value materials and therapeutic models. Sophisticated digitization of discovery workflows, powered by machine learning, relies on versatile, reliable small-molecule templates like this one. Regulators and downstream manufacturers demand ever-cleaner, lower-toxicity building blocks, pushing producers to develop cleaner synthetic methods and minimize residual impurities. Industry trends suggest soon, biotechnological routes to chlorinated heterocycles may eat into traditional petrochemical pathways, setting up new debates about tradeoffs between cost and environmental impact. Each step—whether on the bench or at production scale—brings more attention to life-cycle analysis, recycling, and closed-loop systems, fostering not just technical but responsible innovation.
You don’t hear about 2,3-Dichloropyrazine on the news or see it trending online, but it plays a real part behind the scenes in the chemical world. This molecule, as its name hints, comes from the pyrazine group, with two chlorine atoms added in just the right spots. For most folks, big-sounding chemicals rarely make it into conversation, but for people in research or working with agrochemicals, these compounds matter in a big way.
On the farm, pests and disease can ruin a good season. Over the years, synthetic routes using compounds like 2,3-Dichloropyrazine help chemists build stronger crop protection products. The molecule acts as a building block for new pesticides and fungicides—not ones you’d buy at the hardware store, but the raw material the labs use to invent them. Once combined with other chemicals, it brings certain traits to the table, like stability or the ability to handle tough environmental conditions. The agricultural sector keeps searching for safer, yet more productive ways to guard crops, and the kind of chemistry enabled by intermediates like this one opens new doors.
The pharmaceutical field takes the idea a step further. Researchers start with something like 2,3-Dichloropyrazine to shape novel molecules for experimental medicines. It allows precise placement of atoms and groups, which influences how a potential drug behaves in the body. People chase new antibiotics, antiviral drugs, or treatments for rare conditions, and that often begins with carefully chosen building blocks. I’ve read about several active compounds built from pyrazines—some currently under clinical trials for tough diseases—showing promise because the chemistry lets teams tweak small features quickly before getting to animal tests.
Facts matter here. The chemical supply chain counts on substances like 2,3-Dichloropyrazine to accelerate research. You’ll see patents that mention it in early-stage reaction steps, particularly where researchers try to improve yields or find new treatment paths. Science writers might gloss over the gritty steps, but this is where the real groundwork gets done. Looking for cost savings, consistent supplies, or purer end products? That starts with better intermediates.
A problem pops up when you think about how all this fits together. Chemical manufacturing raises questions about environmental safety, health risks, and exposure during transport. Factories have to control emissions, so waste doesn’t end up in waterways or the air. Folks working in synthesis wear protective equipment, since exposure can irritate skin or lungs. In my years talking to people from industry, I’ve heard worries about regulations getting tighter, or companies outsourcing to countries with lower production costs. We all want cleaner, safer ways to do things. Green chemistry methods—using less hazardous materials, recycling solvents, designing processes that make less waste—make a real difference.
Reliable sourcing and quality controls support the E-E-A-T standards Google values. Peer-reviewed articles and regulatory documents mention this molecule, especially where it serves as a starting material. Chemists rely on safety data, standardized purity, and complete documentation before working at any scale. Anyone in research or industry must keep safety expectations high and keep learning about new alternatives or safer techniques.
You won’t see 2,3-Dichloropyrazine on a shelf at your local pharmacy or garden center. Still, without intermediates like these, both crop protection and drug discovery would crawl. As innovation grows, so does the need for smart, safe, and reliable chemistry—without losing sight of people and the planet along the way.
2,3-Dichloropyrazine has the chemical formula C4H2Cl2N2. In simple terms, this molecule includes four carbon atoms, two hydrogens, two chlorines, and two nitrogens. You won’t find this compound bottled up in corner stores, but researchers and labs regularly cross paths with it. Looking at the actual atoms gives you a small window into how chemistry unlocks real-world innovation, not just classroom trivia.
Years in the lab taught me the value of knowing chemical structures by heart. One day you’re reviewing notes for a new synthesis, and the next, you’re puzzling over a reaction that refuses to cooperate. Sometimes, the issue boils down to a tiny structural detail. Two chlorines on adjacent carbons—the 2 and 3 positions—create a pattern that changes how this molecule interacts with other substances. That matters a lot if you try to fine-tune catalysts or explore new pharmaceuticals.
Chemicals like 2,3-Dichloropyrazine often play a hidden part in more complicated reactions. Drug design teams sometimes look for new ways to outsmart diseases, and the dichloro substitution changes the whole game. That extra kick from chlorine atoms can mean a molecule binds tighter, blocks a pathway better, or resists breakdown inside the body. Even agrochemical research uses small tweaks like these to design safer or more effective pesticides, helping feed the world with fewer side effects.
Using chemicals safely never gets old. Chlorinated aromatic compounds require attention because accidental releases or improper handling can surprise even seasoned professionals. Gloves, vented hoods, and safety reviews become habits. It isn’t just about personal safety; traces escaping from a research bench or a production floor can pollute air or water nearby. Anyone responsible for handling or disposing of chemicals such as 2,3-Dichloropyrazine wants to keep their house in order—knowing its formula helps double-check safety data sheets, plan emergency responses, and support community trust.
Reading chemical formulas tells part of a story. Seeing C4H2Cl2N2 isn’t just reciting numbers—it means remembering that every tiny change in structure might leave a bigger mark when it mixes with the world around us. Training students to check details and respect the power of substitution doesn’t only benefit them; it shields the broader community from unintended consequences. On my university campus, our best teachers would quiz us on why these differences mattered, drilling into our heads that cutting corners on fundamentals never pays off in the long run.
If regulators, researchers, and industry worked closer together, tracking and sharing data on not only well-known substances but those niche intermediates, the chemistry field could march forward with confidence. In labs I’ve worked, double-checking inventory and proper labeling prevented costly mistakes. Tools exist for open reporting on chemical hazards, and it’s practical to use them. By treating even “simple” formulas with respect, chemists set an example for safe, smart problem-solving in a high-stakes world.
2,3-Dichloropyrazine doesn’t sound like something you run into during a trip to the grocery store. This is a chemical used mainly in industrial and lab settings, not everyday household products. Whenever I hear unfamiliar chemical names, my first reaction is curiosity followed by a healthy dose of caution. For decades, many substances popped up in manufacturing, only for folks to find out years later they carried hidden dangers.
Research on 2,3-Dichloropyrazine won’t fill up a public library shelf, but from the data available, there are clear reasons to be careful. Material safety data sheets flag it as harmful if swallowed, inhaled, or if it makes contact with skin and eyes. I’ve seen first-hand how lab workers handle chemicals like this with gloves, goggles, and fume hoods, not because they want to just follow protocol, but because these chemicals cause irritating or toxic effects quickly. 2,3-Dichloropyrazine has a structure similar to other chlorinated compounds, many of which are notorious for irritating mucous membranes and causing respiratory issues.
Folks in chemistry circles know to treat chlorinated organic compounds with respect. Some chemicals in this class are carcinogenic or can trigger ongoing health issues. There’s no broad study on 2,3-Dichloropyrazine and cancer risk, but given what’s known about other related molecules, it’s not wise to get complacent. I’ve read toxicology reports pointing out the risk of organ damage over extended exposure. Short-term, you’ll likely see headaches, dizziness, and skin rash if safety measures lapse.
Many believe chemicals shipped in drums or bottles rarely impact regular folks. Yet, chemicals like 2,3-Dichloropyrazine don’t always stay confined to warehouses and labs. Spill accidents happen. Waste sometimes gets handled poorly. As someone who lived near an industrial site in my twenties, I remember the fear that came with periodic mystery odors drifting through the neighborhood and stories of workers visiting the ER with chemical burns or breathing issues.
It’s not about spreading fear, but about understanding what proper chemical stewardship does for communities. Even trace releases into water, soil, or air can add up, especially near plants or facilities where such compounds are produced or stored. The EPA and OSHA set exposure limits for many chemicals, often after learning the hard way about long-term effects. For 2,3-Dichloropyrazine, the lack of detailed long-term studies means extra vigilance.
Training and protective gear shouldn’t be optional. Lab workers and factory crews need real education, not just a page of instructions, about what they’re handling. Good chemical hygiene—ventilation, gloves, goggles, and regular health checks—helps keep accidents rare and reduces harm when they do happen. Companies ought to share safety data openly and update it as science evolves. Clear labeling and consistent storage keep everyone in the loop.
Regulation can push manufacturers and transporters to invest in safer packaging, tighter handling standards, and improved emergency response. Local communities benefit from transparency and having a say in what sets up shop near their homes. My own experiences taught me that conversations between plant managers, workers, and neighbors promote responsibility better than checklists ever could.
2,3-Dichloropyrazine doesn’t belong in the category of everyday dangers, but neither is it harmless background noise. Whether in a lab or in a town next to a chemical plant, people deserve protection rooted in caution, clear information, and honest engagement. Facts, trust, and respect—for chemicals and for each other—keep communities safer, at work and at home.
A lot of chemists don’t talk much about storage until something goes wrong—stained labels, odd odors, sometimes a leaking cap. After years in research labs, I’ve learned that keeping chemicals like 2,3-Dichloropyrazine in good shape is more than ticking boxes on a safety form. It’s about setting up the right environment, every time, so the risks stay low and the compound sticks around in top condition for your work.
2,3-Dichloropyrazine stays stable at room temperature if the space is cool and out of direct sunlight. Even though it’s tempting to crowd everything onto one shelf, this compound asks for consistency—fluctuating heat can degrade purity. I remember a summer when a lab’s flaky air conditioning meant some stocks fell below spec, and the headaches started with failed reactions and angry emails to suppliers. Reliable air or a temp-monitored chemical cabinet saves money and time, especially when projects run tight on deadlines.
Humidity can turn a reliable chemical into a liability. Moisture sneaks in through loose lids and old plastic, so a sealed glass container with a screw cap works best. I always label containers with date and initials, so there’s no confusion during audits. Silica gel packs aren’t just for electronics—they help inside storage boxes to keep the air bone-dry. Ignoring these basics can bring in clumped powders or mystery solutions, both totally avoidable if the habit sticks to check the seal every time.
Ventilation isn’t a luxury, it’s just as important as lockable doors. I’ve seen small leaks become big headaches because fumes collect and do their own chemistry. Chemical storage that breathes, usually with an extraction system or at least an open-window policy, keeps surprises at bay. It matters who you store chemicals next to—2,3-Dichloropyrazine doesn’t play well with acids or oxidizers. Once, a cross-contaminated shelf ruined both stocks and forced a full cleanout. Always keep it away from those volatile neighbors; separate shelves really do save your budget and your day.
Storing chlorinated compounds isn’t just about spoilage. Fire risk rises when flammable solvents share the space. Flammables cabinet, dedicated signage, no shortcuts. Sprinkle in a routine check: is there a dry chemical fire extinguisher on hand and does everyone know how to use it? You never want to answer that question in a frantic moment, so dry runs make sense. Labs run best when nobody second guesses emergency stops or routes out the door.
Documentation isn’t glamorous, but it’s real protection. Without written proof—supplier lot number, receipt date, condition notes—problems turn into finger-pointing. I like old-school logbooks paired with digital tracking so nothing slips through. Once, this backup system helped us pass a surprise inspection without a scramble. Every container checked and restocked before it turns dodgy keeps labs safe and experiments honest.
Smart storage for 2,3-Dichloropyrazine isn’t about fancy equipment; it rests on routines. Cool, dry, tightly sealed, and never next to reactive chemicals. Always remember to check labels, update logs, and test the air. Protecting your chemicals means protecting your people and your science—trusting a system set up right from the start. That’s experience talking, not just policy on paper.
People in labs, universities, and factories have to deal with numbers every day. Some numbers just keep coming back, like the molecular weight of chemicals. For 2,3-Dichloropyrazine, that number is 147.97 grams per mole. It can look bland on paper, yet this value does a lot of heavy lifting in real-world situations.
A big part of my early chemistry classes revolved around measuring stuff out—calculating how much of a powder or liquid to use, trying to get experiments to work without wasting any chemicals. You can’t weigh out the right amount or prepare solutions with the right concentration unless you know the molecular weight. It grounds everything in the lab. One mistake and the results get weird, waste goes up, and the work sometimes has to start over from scratch.
Think of a synthetic route where 2,3-Dichloropyrazine comes in as a starting material or an intermediate. Reactors have limits. Lab budgets have limits. Even accuracy has a limit, and that’s where the molecular weight keeps people honest. Someone planning a reaction on a bigger scale can’t just wing it—they need solid numbers for every gram, and the molar mass provides that precision. Too little, you miss your target. Too much, the system goes out of balance or blows your safety margins.
Teachers and students both lean on these fundamentals. Students who once forgot to factor in molecular weight on a test quickly learned not to skip it again. Multiplying moles and grams gets old after a while, but there’s no shortcut for precision. One of my chemistry professors liked to say, “Numbers don’t lie, but ignoring them will trip you up.”
Handling something like 2,3-Dichloropyrazine, which has two chlorine atoms on a pyrazine ring, means respecting the properties that come with it. Chlorine atoms add heft to molecules—each atom brings 35.45 grams per mole. Those extra weights increase the material’s molecular weight and often tweak toxicity and handling rules. Safety data sheets take these things into account. Chemists planning hazard controls or emergency responses look at how much substance could vaporize or spill, and the calculations stem from molecular weight. Getting this wrong could mean underestimating exposure or missing a potential risk.
On a larger scale, manufacturing and quality control rely on exact measurements of reagents. The price tag on 2,3-Dichloropyrazine doesn’t just depend on supply or demand—it builds from the efficiency of each batch run, and every calculation ties to molecular weight. Margins in chemical production squeeze tighter every year. Accuracy helps save money and cuts down on waste, which counts for both the bottom line and environmental sustainability.
People trust labs and manufacturers that consistently report reliable numbers in their documentation. Using accurate molecular weights in COAs (Certificates of Analysis) and publications adds to that trust. Mistakes risk not just faulty research but reputations, future grants, and partnerships. Chemists and suppliers, from personal experience, have to double and triple-check numbers, compare them across sources, and draw on resources like trusted textbooks, reliable databases, and direct measurements when possible.
So while the molecular weight of 2,3-Dichloropyrazine—147.97—starts as a dry fact, it grows roots through every part of chemistry from bench work to policy. It’s a simple square on the periodic table that quietly shapes safer experiments, sharper business, and better science.
| Names | |
| Preferred IUPAC name | 2,3-dichloropyrazine |
| Other names |
2,3-Dichloropyrazin Pyrazine, 2,3-dichloro- NSC 408173 |
| Pronunciation | /ˈtuː ˈθri daɪˌklɔːrəʊpaɪˈreɪziːn/ |
| Identifiers | |
| CAS Number | 17636-42-1 |
| Beilstein Reference | 120993 |
| ChEBI | CHEBI:39441 |
| ChEMBL | CHEMBL330207 |
| ChemSpider | 19745 |
| DrugBank | DB08336 |
| ECHA InfoCard | 03a9e351-9346-4a6a-a9f0-9e47e5d9b55c |
| EC Number | 210-765-6 |
| Gmelin Reference | 570982 |
| KEGG | C14343 |
| MeSH | D004082 |
| PubChem CID | 13945 |
| RTECS number | UG4375000 |
| UNII | 77PY14DCCF |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | DJ8702000 |
| Properties | |
| Chemical formula | C4H2Cl2N2 |
| Molar mass | 147.00 g/mol |
| Appearance | White to yellow powder |
| Odor | Odorless |
| Density | 1.485 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 1.6 |
| Vapor pressure | 0.15 mmHg (25 °C) |
| Acidity (pKa) | 1.12 |
| Basicity (pKb) | 2.02 |
| Magnetic susceptibility (χ) | -60.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.521 |
| Viscosity | 1.015 mmHg (25°C) |
| Dipole moment | 1.74 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | S°298 = 322.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -8.1 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1077.4 kJ mol⁻¹ |
| Hazards | |
| Main hazards | Harmful if swallowed or inhaled. Causes skin and eye irritation. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P273, P280, P301+P312, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | 90°C |
| Autoignition temperature | 620°C |
| Lethal dose or concentration | LD50 oral rat 3200 mg/kg |
| LD50 (median dose) | LD50 (median dose): 2000 mg/kg (rat, oral) |
| NIOSH | SN4000000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.1 ppm |
| Related compounds | |
| Related compounds |
2,6-Dichloropyrazine 2,5-Dichloropyrazine 2,3,5-Trichloropyrazine 2-Chloropyrazine 3-Chloropyrazine |