Chemists looking for new building blocks in the twentieth century gave 2,3-dibromothiophene more attention after discovering how useful substituted thiophenes can be for synthesizing pharmaceuticals and materials. Researchers in the 1960s found that halogenation of thiophene offers a pathway toward many novel compounds. The bromination of thiophene using elemental bromine or milder reagents followed established patterns from classic textbooks, but interest in the 2,3-isomer picked up because its unique substitution pattern opens different routes for further functionalization. Academic circles frequently referenced this compound in discussions on electronic effects within heterocycles. Today, 2,3-dibromothiophene doesn’t often appear in household chemistry sets, but among those investigating organic electronics or certain agrochemicals, it represents a familiar starting point.
2,3-Dibromothiophene slots firmly into the group of organosulfur heterocycles with two bromine atoms tagging the thiophene ring at the 2 and 3 positions. It is a colorless or pale yellow liquid, often chosen for targeted synthesis rather than bulk chemical production. I recall its sharp, distinctive odor, which stands as a warning to handle with care in the lab. Chemical suppliers offer it to research labs and industry, sometimes in small amber bottles to shield it from light and preserve its integrity. Scientists order it for precise research aims, not for mass manufacture or household tasks, so you don’t find it on the shelves of standard supply stores.
With a molecular formula C4H2Br2S and a molar mass tipping the scale at about 242 grams per mole, 2,3-dibromothiophene looks unassuming but holds some unique cards. The presence of two heavy bromine atoms increases the density above many other thiophene derivatives, and it boils around 230 to 232°C. It mixes only modestly with water, but you’ll see it blend easily with most organic solvents—ether, chloroform, and acetone all do the trick. Electronegative bromine atoms draw electron density away from the ring, tweaking its reactivity toward nucleophiles. Its refractive index sits slightly higher than pure thiophene, which sometimes helps researchers differentiate it in the lab.
Technical data sheets reflect the needs of chemists who push for high purity. Most suppliers list it at 95% minimum purity, sometimes higher, with GC analysis provided as proof. Labels clearly display its CAS number, 1511-96-2, and sometimes the EC number 216-142-3. You’ll see recommended storage at low temperature, away from direct sunlight and oxidizers, since both can lead to gradual decomposition or discoloration. Packaging instructions caution against glass-to-glass contact, since brominated organics sometimes react slowly with some glassware. Even experienced researchers check the label twice thanks to the many similar names floating around in the lab inventory.
Historically, bromination of thiophene in acetic acid, carbon tetrachloride, or dichloromethane created the first samples of 2,3-dibromothiophene. The reaction runs at room temperature or slightly above, with bromine added dropwise to control reaction rate, since excessive heat stirs up side reactions. These days, alternatives like N-bromosuccinimide (NBS) serve as a milder, more selective brominating agent. Filtration, washing, and careful distillation yield a purified product. Having run similar halogenations, I know that ventilation matters; the fumes cloud the air and leave your eyes stinging, so fume hoods and gloves always come out before opening a bottle of bromine.
Two bromo groups make this molecule a prime candidate for substitution reactions, especially metal-halogen exchange and Suzuki or Stille coupling. Chemists strip off one or both bromines, swap in various substituents, and construct new rings or polymers. That opens a path to complex intermediates in pharmaceuticals and the building blocks of organic electronics. For example, I’ve seen researchers toying with different palladium-catalyzed couplings to build longer conjugated systems, using 2,3-dibromothiophene as a backbone. Because the reactivity at the 2 and 3 positions is predictable, results stay consistent across different research groups, which speeds up the trial-and-error process. The compound survives most gentle reductions, but strong bases or nucleophiles can nick the ring.
Over the years, this compound picked up a list of alternate names: 2,3-dibromothiophene appears as Dibromothiophene, 2,3-; 2,3-Dibromo-1-benzothiophene; and sometimes slips into catalogs as 2,3-thiophenedibromide. Each name points chemists to the same structure, though in practice, the CAS number keeps supply clerks and researchers from grabbing the wrong bottle. Some niche companies package it under trade names or abbreviations, but the most accurate conversations stick to the IUPAC name or CAS number to prevent mistakes in synthesis protocols.
Lab safety rules flag this compound for several concerns. Inhalation, skin contact, or accidental ingestion all carry risk, since brominated compounds often irritate mucous membranes and can spark allergic reactions. In my own lab, the safety data sheets tell us to use goggles, gloves, and a fume hood whenever handling 2,3-dibromothiophene, and never to pipette by mouth, no matter how pressed for time. Dilute spills need careful mop-up using absorbent pads, while larger releases demand evacuation and rescue team support. The waste goes in halogenated organic containers, far away from acidic or alkaline waste, since secondary reactions could pose fire or release toxic gases. Emergency showers and eyewash stations matter more for compounds like this, and I’ve seen supervisors insist on regular refreshers for staff who handle brominated chemicals.
Custom synthesis builds on the bromo groups at positions 2 and 3, placing 2,3-dibromothiophene right in the middle of organic electronics and pharmaceutical R&D. I’ve watched colleagues prepare it as a precursor for new conductive polymers, semiconducting films, or organic light-emitting diodes (OLEDs). Pharmaceutical researchers aim to swap the bromo groups with different side chains to find new drug candidates with unique properties. Agrochemical teams look for novel crop protection agents by modifying the thiophene ring. In universities, it helps as a model compound to teach substituent effects on aromatic systems in advanced organic chemistry labs. Its value sits in its adaptability and the predictable behavior of its reactive positions, making experimental planning less guesswork.
Development teams continue seeking better brominating agents to control substitution patterns or avoid unwanted by-products. Analytical chemists refine methods for NMR, mass spectrometry, and chromatography to track impurities at single-digit ppm levels. Synthetic chemists look to couple 2,3-dibromothiophene into larger, more complex structures—think polythiophenes for flexible displays or biosensor arrays. A few teams work on modifying the ring to tune electronic properties for specialty applications. I’ve seen efforts to prepare chiral derivatives that could play a role in enantioselective synthesis or catalysis.
Toxicity often becomes a limiting factor in chemical innovation. Animal studies on 2,3-dibromothiophene highlight mild to moderate toxicity, with exposure leading to skin, eye, and respiratory tract irritation. Chronic effects haven’t been studied widely, but related dibrominated compounds sometimes show environmental persistence and bioaccumulation, especially in aquatic systems. Regulatory guidance remains sparse, so many research groups treat it as hazardous by default, using the same level of caution reserved for higher-profile toxicants. Waste management practices try to catch all residues, since it could pose hazards downstream if poured down the drain or left in regular trash. If used in consumer-facing products, more thorough studies on long-term toxicity and breakdown need to be run.
Looking ahead, interest won’t dry up as long as chemists need versatile synthons for polymer and medicinal chemistry. As green chemistry grows in importance, researchers look for new, less hazardous ways to introduce bromine onto thiophene rings, or even to bypass brominated intermediates altogether. If regulators restrict brominated substances or if novel, safer coupling components emerge, fields that use 2,3-dibromothiophene could shift. Researchers continue refining large-scale synthesis techniques to cut down on waste and avoid excess hazards, aiming for both safety and sustainability. As electronics miniaturize and pharma hunts down new molecular scaffolds for drugs, the reactivity provided by doubly brominated thiophenes remains tough to beat. For anyone who has worked in a lab, the value of a reliable, well-characterized intermediate never fades, especially as the next generation of researchers takes up the challenge to make new materials and medicines that work better, last longer, and harm less.
I’ve always seen chemistry as a puzzle, one where the pieces don’t just fit, but they tell a story about how things come together and what kind of magic happens when molecules meet. Take 2,3-dibromothiophene, for example. Its name might sound like something a comic book villain conjures, but it’s a real player in the world of organic synthesis and electronic materials.
2,3-Dibromothiophene has a pretty straightforward chemical formula: C4H2Br2S. Those little numbers matter. They speak to chemists about what’s hanging onto that core ring structure. Thiophene itself is a five-membered ring—four carbons, one sulfur. Add in the two bromines at positions two and three, and you get a molecule that looks simple on paper but packs a punch in the lab.
Let me break it down like this: thiophene forms the backbone, giving the molecule stability and its aromatic character. Bromine atoms, added at the second and third positions, bring new reactivity and potential for creating more complex molecules. Lab folks chase after that kind of structure because it opens gates to all sorts of functional materials.
The real magic in a formula like C4H2Br2S comes out in what it can do. Chemists use 2,3-dibromothiophene as a building block. You see it in the synthesis of organic semiconductors and molecular electronics, places where rapid development isn’t just about speed—it’s about precision and reliability.
Some folks might not think molecule substitutions matter much, but those substitutions turn theory into reality. Electronic screens and flexible displays lean heavily on molecules that behave just right when a little charge runs through them. A small tweak like two bromine atoms on a thiophene can lead to entirely different electrical properties.
I’ve worked alongside both academic and industry researchers who push for better starting materials. While 2,3-dibromothiophene can offer access to next-gen polymers, handling brominated compounds brings safety questions and waste concerns. People who spend hours hunched over lab benches know that while innovation moves fast, safety practices lag if ignored. Smart engineering controls, fume hoods, and careful storage keep danger at bay but often get overlooked when deadlines pile up.
Waste management sits right behind synthesis on the priority list. Bromine runoff and disposal need more than a shrug. Cities downriver of industrial centers shoulder the risk when those chemicals slip through. It rings true that proper chemical stewardship means factoring in downstream impact, not just yields and papers published.
There’s a lot to like about the progress stemming from molecules like 2,3-dibromothiophene. Yet, chasing greener synthesis and sustainable scale-up sits at the core of modern chemistry discussions. Process chemists I know always swap stories—how a tiny tweak to a reaction saved money, reduced hazardous byproducts, or made the process less toxic.
In a lab or a meeting room, hearing that a leap in discovery ties back to one solid, well-understood building block, makes you appreciate how much is riding on those atoms and bonds. Getting that formula right—C4H2Br2S—is as much about understanding as it is about progress.
I spent years in the university’s organic chemistry wing, elbows deep in glassware and reaction logs. Back then, it was clear that 2,3-Dibromothiophene often ended up on the shelf whenever researchers set out to make new molecules for materials science and medicine. The structure—two bromines attached to a thiophene ring—looks simple, but this chemical really packs a punch as an intermediate.
Let’s break down why it attracts so much attention. Unlike more common aromatics, thiophene rings bring sulfur into the picture. Add a couple of bromine atoms, and suddenly the whole molecule becomes highly reactive in the best way. Many specialists reach for it because they need to swap out those bromines for something else—silicon, nitrogen, fancy chains, you name it. That gives chemists a reliable building block they can nudge in dozens of directions. For someone who’s spent evenings planning synthetic routes, finding such versatile stepping stones is invaluable.
Thiophene-based pieces show up everywhere in electronics. If you’ve got a flat-screen TV or a phone with punchy colors, there’s a solid chance a thiophene finds its way into the organic layers that light up each pixel. Labs turn to 2,3-Dibromothiophene as a way to stitch longer, more complex thiophene chains. These chains get used to craft organic semiconductors—stretchy, lightweight materials that move electric charge without metal. Modern OLED screens rely on these materials to stay efficient and vibrant.
The journey from raw chemicals to a working display might look out of reach for most people, but the groundwork starts with practical compounds like this dibromo derivative. Researchers always look for ways to hook different fragments to a thiophene core, tuning the brightness, lifespan, and energy use of new devices. More than once, I watched a technician in my lab light up a tiny test screen for the first time, energized by the success of a new synthesis.
The pharmaceutical field likes to keep new molecular scaffolds on hand. Sulfur-rich rings have shown promise in biological systems, especially when they bind to chemical targets in the body. 2,3-Dibromothiophene lets medicinal chemists test different attachments in search of better drugs—something faster-acting, or maybe less likely to provoke an allergic reaction. Screening analogs isn't just about theory; it demands hands-on work with chemical libraries, and dibromo building blocks take center stage in this process.
Beyond medicines, these thiophene backbones help craft biosensors. Since the sulfur in thiophene can interact specifically with metals or proteins, scientists use this as a starting point for probes and detection tools. In my lab, we made simple sensing strips that would change color in the presence of mercury, relying on reactions unlocked by the dibromo starting material. This helped us keep an eye on heavy metal contamination in water—a concern more households face than you’d think.
Handling brominated chemicals means working with care. The hazards can’t be ignored—mask, gloves, and fume hoods need to stay by your side. The move toward “greener” chemistry pushes us to find processes that cut down on waste and hazard. Some teams use flow reactors, which keep reaction volumes small and help contain spills. Others explore using alternative, less toxic reagents, but for now, 2,3-Dibromothiophene remains a staple in the toolkit.
In day-to-day research, these small rings quietly open doors—whether that’s improving a television’s glow, crafting a new drug, or chasing traces of lead in our water. As someone who’s leaned over the benchtop late at night, it feels good knowing that modest chemicals like this one still find a way to shape the future of technology and health.
2,3-Dibromothiophene isn’t something you toss in a cabinet and forget. This compound, used in research and specialty synthesis, brings a few risks if shrugged off. Getting storage right matters. Most labs keep volatile or reactive chemicals isolated for a reason. This chemical sits in the same group—unpredictable in the wrong spot.
Anyone who’s worked with brominated compounds knows the fumes mean business. Short exposure leads to headaches, coughs, even worse if you skip the fume hood. So, 2,3-Dibromothiophene calls for a tight seal. Glass bottles with PTFE-lined caps do the trick. Metal reacts, plastic sometimes splits. That leaves glass, tucked in a cool, dry place—a dedicated chemicals fridge or a ventilated cabinet. Stack other bottles alongside, and you gamble with cross-contamination. One spill triggers cleanup duty for the whole bench.
Manufacturers slap on hazard icons for more than paperwork. 2,3-Dibromothiophene often carries both flame and toxic warning labels. This isn’t just rules—brominated aromatics can spark up if you store them next to oxidizers or toss them near heat sources. It’s not just inconvenience; it’s life and limb. I’ve seen serious burns from careless storage, and the guilt never fades. Keep ignition sources away. Use spark-free equipment if you’re handling larger bottles or working in bulk.
Those tight seals matter for another reason. This stuff lets off fumes, and it eats through certain plastics over months. No one wants a slow leak in a cabinet—by the time you smell it, the mess has spread. Avoid storing near acids, bases, or anything with cyanide. Brominated thiophenes stir up surprising reactions that nobody wants to see firsthand. Proper labels go far; write the date you open each container to track age. After a couple years, disposal beats risky use every time.
A lot of chemicals looked manageable on paper, only for a small mistake to bring regret. Gloves by themselves don’t solve it. Nitrile or neoprene hold up better than latex, but fumes slip past if you breathe unfiltered air. Sometimes someone works under the impression that working fast solves the problem—but vapor builds anyway. Fume hoods and lab coats matter with 2,3-Dibromothiophene. That’s not paranoia—skin contact brings irritation, and a direct splash raises the stakes.
A buddy system goes a long way. Anyone handling reactive or potent organics should work with someone nearby. Emergencies pop up faster than most expect. Chemistry departments get safety drilled in from day one, but in smaller labs, routines slip. A spill kit and an eyewash station need to be close enough to matter. Learn where they sit before you open the bottle; don’t wait for an accident.
Rules start with storage and handling, but culture cements good habits. Regular safety checks and refresher training keep safety real—not just paperwork. Inventory matters. Expired or unlabelled bottles should leave the shelf quickly. Less clutter means fewer accidents. A little paranoia keeps the workplace healthy.
Lab work always balances curiosity with caution. Respect for 2,3-Dibromothiophene and any brominated compound shows in the routine—the right fridge, a sealed bottle, and no cutting corners with protective gear. Others in the space feel safer, and you work with a little less worry.
When people hear about chemicals with names like 2,3-Dibromothiophene, most look for quick answers: Is this stuff dangerous? Might it hurt people at work, or leak into places it shouldn’t? The short answer is that brominated compounds often come with red flags, but let’s dig in with details and some real perspective.
2,3-Dibromothiophene isn’t something you’ll bump into at the grocery store. It’s a lab chemical, mostly popping up when people work in organic synthesis or chemical research. Because of its structure—a thiophene ring loaded with two bromine atoms—it falls into a broad group that sometimes brings trouble. Bromine itself has a reputation for making molecules more reactive and more likely to irritate humans and mess with natural systems.
I once worked summers in an academic chemistry lab, so I know the sharp, sometimes choking smell of bromine derivatives. Protective gloves and strong ventilation felt like common sense, not just rules printed in a book. Colleagues older than me stayed wary of things that could sting skin, burn lungs, or linger on shared equipment if spills happened. You learn quickly that even brief exposure to brominated stuff can leave rashes, breathing problems, and eye pain—not something you want to repeat.
Official safety sheets flag 2,3-Dibromothiophene as hazardous. The word “toxic” appears on most supplier paperwork, with warnings about possible harm to the liver and kidneys based on animal studies. Direct evidence in people stays scarce, but that doesn’t make the risks disappear. Volatile chemicals get into the air and can stick to surfaces. Labs need washing stations, gloves, and goggles for good reason. Even in small quantities, dust or vapor from these compounds sneaks past open containers, and repeated exposure is what really adds up over a career.
What goes down a drain ends up somewhere. Persistent organic chemicals like 2,3-Dibromothiophene do not just break down overnight. Brominated bits stay in the soil or water, and small creatures end up carrying the burden. Studies on similar compounds show tendencies to bioaccumulate and drift up the food chain. Birds, fish, and even humans take in tiny doses over time, and nobody wants to be the guinea pig for unexpected effects.
Large chemical plants and university labs have clear-cut procedures, but slip-ups still occur. I have seen gloves tossed without care and spills ignored with a paper towel. Culture grows from hands-on training and open conversations about accidents, not just printout reminders or thick binders in the back room. Young chemists should get a blunt overview of the worst-case scenarios. Shortcuts seem tempting in the rush of deadlines, but stories about old accidents and health scares stick long after graduation.
Alternatives do exist, though. Some organic syntheses can happen with less toxic reagents, ironically cutting costs once labs consider cleanup and disposal headaches. Routine air sampling, proper labeling, strict segregation, and end-of-shift decontamination can slice risks way down.
2,3-Dibromothiophene might seem obscure, yet the challenges around it echo across the whole world of brominated chemicals. Small steps—dust masks in the right spot, careful disposal, strict inventory—build up to a safer environment. People deserve honesty about what they’re working with and why precautions never go out of style, because chemistry—like most jobs—always gets safer when experience and facts shape every step.
Most folks in labs don’t want to worry about what’s actually in a reagent bottle, but with 2,3-Dibromothiophene, a staple in many synthetic routes, you soon find out that not all suppliers play by the same rulebook. Purity isn’t just some fine print thrown on a spec sheet—it’s the difference between a project moving forward or someone scratching their head for weeks.
My own rough lab notes from grad school tell the story: suppliers usually list this compound anywhere from 97% to 99% pure. Some go as low as 95% for bulk or industrial batches, especially in markets where cost takes the lead. Folks working high-stakes organic synthesis, especially in pharma or dye design, usually demand the 99% variant. They’ve learned that lower grades often carry the ghost of dibrominated side products or leftover elemental bromine. Suddenly, yields drop, NMR peaks blur, and there's a midnight run to find another supplier.
Standard 2,3-Dibromothiophene batches don’t just bring the main act. Byproducts linger, like unreacted thiophene, over-brominated versions, leftover catalysts, and sometimes moisture that sneaks in during shipping. If you’ve ever smelled a freshly opened bottle and caught a whiff sharper than expected, you’ve probably just met a trace of free bromine. Fiddling with that is not only unpleasant—it's a safety risk. Sodium thiosulfate helps, but most buyers would rather not mess with unexpected clean-up jobs.
Some folks argue a small impurity isn’t much in exploratory chemistry, but real experience says otherwise. One research group I worked with spent weeks troubleshooting sluggish cross-coupling reactions, only to trace the problem back to a dodgy reagent lot with just 96% stated purity. Their HPLC traced a handful of peaks below the threshold, but they killed selectivity in the end product. That kind of headache gets expensive fast, both in time and actual cash.
Reading a supplier’s certificate of analysis often feels like squinting at fine print. Only a handful spell out impurity levels beyond a simple “97% GC.” Even then, GC doesn’t catch everything, especially if you’re worried about stuff like organometallic contamination or persistent solvents. Top-tier chemical houses sometimes toss in NMR or HPLC data, but cost ticks up, and not everyone bankrolls that luxury. I’ve sent samples for third-party analysis more than once, and a surprising number fall short of label claims. Nobody likes a surprise, especially after you’ve got $5,000 worth of material in hand and a synthesis on the clock.
High-end applications like pharmaceuticals, materials R&D, or sensor tech place the spotlight on impurities you might otherwise brush off. The rest of us chasing early-stage results sometimes risk it with off-the-shelf grades. As projects mature and scale looms, headaches from batch-to-batch inconsistency become more than tiny annoyances—they turn into phone calls with suppliers and endless paperwork. I’d rather invest upfront in certified high-purity lots than bet my work on hope and luck, though that’s a luxury not every budget offers.
Solving the mystery takes honest supplier relationships and open communication. I always ask for detailed batch analysis, push for transparency on purification steps, and skip vendors who dodge questions. Some producers go the extra mile with digital batch records or third-party lab verifications. I follow up with in-house checks: a good NMR and sometimes single-crystal X-ray if things don’t add up. You can never rule out surprises, but some diligence beats weeks of troubleshooting rotten chemistry.
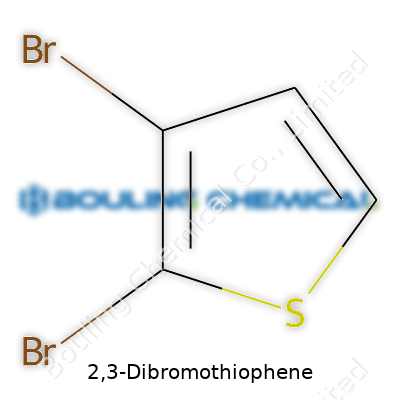
| Names | |
| Preferred IUPAC name | 2,3-dibromo-1-benzothiophene |
| Other names |
2,3-Dibromo-thiophene Thiophene, 2,3-dibromo- 2,3-Dibromothiofene |
| Pronunciation | /ˈtuː θri daɪˈbroʊmoʊˈθaɪoʊfiːn/ |
| Identifiers | |
| CAS Number | 1462-32-6 |
| 3D model (JSmol) | `3D1w1eUBeQTsgEYxNw6CNdSBgEDDHGgAYQGQYgAy1gVoMwCGBwMwmig9GQAypt5p4YQA` |
| Beilstein Reference | 1209862 |
| ChEBI | CHEBI:38485 |
| ChEMBL | CHEMBL514481 |
| ChemSpider | 15410747 |
| DrugBank | DB08636 |
| ECHA InfoCard | 100.021.384 |
| EC Number | 211-273-0 |
| Gmelin Reference | 83229 |
| KEGG | C18925 |
| MeSH | D003967 |
| PubChem CID | 12545 |
| RTECS number | XN8575000 |
| UNII | 2VHX4A194B |
| UN number | UN1993 |
| CompTox Dashboard (EPA) | DTXSID6071595 |
| Properties | |
| Chemical formula | C4H2Br2S |
| Molar mass | 255.93 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | Strong odor |
| Density | 1.992 g/cm³ |
| Solubility in water | Insoluble |
| log P | 1.8 |
| Vapor pressure | 0.0086 mmHg (25 °C) |
| Acidity (pKa) | -1.63 |
| Basicity (pKb) | 7.12 |
| Magnetic susceptibility (χ) | -81.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.621 |
| Viscosity | Viscous liquid |
| Dipole moment | 1.95 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 362.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | 77.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -414 kJ mol⁻¹ |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin irritation, causes serious eye irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302 + H312 + H332: Harmful if swallowed, in contact with skin or if inhaled. |
| Precautionary statements | P210, P264, P280, P301+P312, P305+P351+P338, P337+P313, P501 |
| NFPA 704 (fire diamond) | 2,3-Dibromothiophene: 2-2-0 |
| Flash point | Flash point: 66 °C |
| Autoignition temperature | The autoignition temperature of 2,3-Dibromothiophene is "410°C". |
| Lethal dose or concentration | LD50 (rat, oral): 400 mg/kg |
| NIOSH | SN8750000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.1 ppm (1 mg/m³) |
| Related compounds | |
| Related compounds |
Thiophene 2-Bromothiophene 3-Bromothiophene 2,3,5-Tribromothiophene 2,3,4,5-Tetrabromothiophene 2,5-Dibromothiophene |