Chemistry often follows a winding path, and 2,3-Diaminophenazine reflects that. Decades ago, phenazine derivatives caught the eye of researchers hunting for flexible dye molecules and bioactive compounds. It didn’t take long for folks to realize how modifications to the phenazine core could crank up its function in everything from textile coloring to biological assays. The gradual transition from laboratory curiosity to research staple happened in lock-step with a better understanding of electron-rich aromatic systems. The chemical’s rise paralleled advances in synthetic organic chemistry, where new routes and milder conditions gave researchers control over multi-step reactions and substitutions. The entry of 2,3-diaminophenazine into the chemical catalog reflects a time when science leaned on persistent effort rather than overnight leaps.
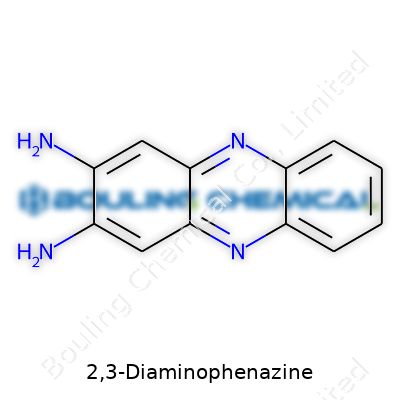
The bright orange-red shade of 2,3-diaminophenazine signals a whole lot more than simple color. In solid form, it comes across as a fine crystalline powder. Its stability under ordinary conditions means it stores easily, which definitely makes a chemist’s work less stressful. Most reference materials catalog it under the CAS Number 5118-45-4, and its molecular formula—C12H10N4—keeps things tidy for those cataloging supplies. A bottle on a bench stands as a launchpad for more advanced research, but even in small quantities, this compound packs enough chemical personality to serve as a building block, sensor, or analytical standard.
You can spot it thanks to that signature reddish color. It doesn’t dissolve well in water, which nudges people toward organic solvents like DMSO, ethanol, or dimethylformamide for both experimentation and processing. Its melting point, hovering around 315-318°C, points to strong intermolecular interactions, hinting at the stability of its planar aromatic system. The presence of amino groups in the 2 and 3 positions turns 2,3-diaminophenazine into a bit of a chemical lynchpin. Those amines offer up hydrogen bond donors, and they open the door to derivatization. Under UV or visible light, it’s possible to observe distinct absorbance features—an advantage for researchers working in photochemistry or analytical chemistry, where sensor and probe development keeps ramping up.
On the technical side, suppliers break down the content by purity (usually >98%), physical description, and a batch-associated lot number. Labels often feature the molecular weight of 210.23 g/mol. Storage guidance generally calls for a cool, dry place away from light—nothing outlandish, but lab managers keep an eye on this to prevent any slow degradation. Some suppliers attach a hazard warning (irritant or harmful if swallowed or inhaled), so gloves, goggles, and ventilation score high for safe practices. Those Material Safety Data Sheets (MSDS) read like commandments, but anyone accustomed to handling aromatic amines won’t find surprises here.
Synthesis often starts with phenazine or its N-oxide derivatives, followed by selective nitration and reduction steps. For example, a traditional route might involve dinitrophenazine, which then undergoes catalytic hydrogenation using a palladium or platinum catalyst to reduce those nitro groups down to amines. Using milder reducing agents, like tin(II) chloride in hydrochloric acid or even sodium dithionite under aqueous conditions, can lower the risk of over-reducing or decomposing the phenazine core. Some labs work with chemo-enzymatic tactics for cleaner yields and fewer byproducts, but it takes careful attention to conditions (temperature, solvent choice, reaction time) to keep purity high. I’ve found working through a pilot-scale batch runs smoother once you dial in the ratio of reagent to substrate and keep a vigorous stirring regime.
Thanks to those two amino groups, modification possibilities spring up. Chemists can acylate, alkylate, or sulfonate the molecule using pretty straightforward protocols. Derivatization enhances solubility or introduces functional groups tuned for sensing, conjugation, or redox chemistry. In redox cycling systems, 2,3-diaminophenazine can act as a mediator shuttling electrons between donor and acceptor. Photochemical reactions explore its utility in organic electronics and catalysis. Understanding its reactivity in the presence of various electrophiles or under oxidative conditions paves the way for new material or sensor platforms. Based on my own bench work, small tweaks in the substituents or protecting groups around the amino functionalities change both its color and electron transfer capacities—useful hacks for anyone building custom reagents.
The compound pops up under a short list of aliases: 2,3-diaminophenazine, 2,3-PDA, and even simple registry numbers like NSC 407068. Chemists sometimes shorthand it in reaction notes as “diaminophenazine” or “DAP” (though that gets confusing among other diaminophenazine isomers). Labeling conventions often include its full IUPAC name (2,3-diaminophenazine) and the CAS number for ordering or inventory systems. I’ve also seen it listed as NCI C05688 in some cancer research supply caves, which sometimes throws newcomers trying to link literature to a purchase order.
Aromatic amines have a history of health risks—think mutagenicity and skin sensitization—so keeping exposure low matters. Ventilated hoods, nitrile gloves, and long sleeves serve as basic defenses. I stick to disposable pipettes and weigh boats, since clean-up is easier and reduces contact risk. Getting in the habit of logging spills or near-misses helps spot trends—maybe a fume hood airflow issue, or sloppy technique during weighing. Regulatory circles call for routine risk reviews, not just with pure substances but during any downstream product development. Disposal through approved chemical waste routes avoids environmental harm, since these compounds break down slowly and could linger if they hit municipal systems or soil.
Diverse application fields keep 2,3-diaminophenazine in circulation from year to year. It plays major roles as a precursor for synthetic dyes and pigments, with deep, stable colors that appeal to both textile chemists and staining specialists in pathology. The compound’s electrochemical properties support sensor and battery research—anywhere electron transfer and color changes help readout or detection. Working with bioassay developers, I’ve seen the molecule used as a substrate in oxidase-based tests: in the presence of certain enzymes, the compound undergoes a colorimetric change that is easy to track visually or with spectrophotometers. Its reactivity also drives its use in tuning redox properties of new polymeric materials, and in organic electronics research chasing flexible and low-cost semiconductors.
The past decade brought a surge of new papers probing subtle modifications to 2,3-diaminophenazine’s core—especially functionalization at the periphery to introduce affinity tags, photoreactive arms, or bioconjugation handles. Groups working on antimicrobial agents tinker with phenazine skeletons, searching for potent allies against resistant pathogens. Electrochemists test its utility in rechargeable batteries and capacitors, eyeing the stable redox window and the chance to customize capacity. Material scientists incorporate its analogs into polymer films or nanoparticles, chasing tailored photophysical and redox behavior. Working in academic and industrial spheres, I’ve noticed small breakthroughs often rely on tweaking the molecule with new substituents, echoing an organic chemist’s innate curiosity to make something just a bit better or different.
Concerns about toxicity run deep for phenazine derivatives, so new research pays special attention to mutagenic and cytotoxicity profiles. Ames tests and in vitro cytotoxicity assays form the backbone of early safety checks, while in vivo models help flag issues that bench work might miss. Some analogs show DNA intercalation or inhibit mitochondrial function in cell studies, tempting those studying cancer but worrying those thinking of broader chemical safety. Following up on long-term exposure studies, regulatory agencies push for better documentation and safer practices, especially if a compound might migrate from research to manufacturing. As someone who’s reviewed MSDS documents for new phenazine relatives, I know keeping a detailed track of exposure symptoms and outcomes shapes how future safety protocols develop in both academic and industrial labs.
Looking forward, 2,3-diaminophenazine seems far from finished. New paths keep emerging in material science, sustainable electronics, and biomedical assay development. Trends in green chemistry stimulate interest in cleaner, more modular synthesis, either leveraging biocatalysis or milder chemical approaches. Research teams bet on hybrid materials linking phenazines to nanostructures or smart polymers for responsive sensors and optoelectronic devices. It wouldn’t surprise me to see next-gen batteries or kits for rapid medical testing built around some clever tweak to the core structure. As regulations get tighter, demand for non-toxic modifications and more complete toxicity screens keeps the field grounded and responsive, keeping one eye on safety and the other on groundbreaking new applications.
2,3-Diaminophenazine isn't a name you'll catch in everyday news. Most folks haven't heard of it, unless they've spent time poking around in chemistry labs, working on research projects, or hunting for ways to detect diseases. What sparks curiosity is how this yellow-orange powder plays a part in shaping new tools behind the scenes in health and science.
The most direct thing you’ll notice about 2,3-Diaminophenazine is its rich color. That pigment isn't just for show — it’s a big part of its usefulness. Chemists use it as a dye in staining processes, which helps people actually see what's going on when looking at cells under a microscope. That color lights up spots where proteins or other targets gather, making it easier to track changes and patterns during experiments.
Years ago, manufacturing test strips for checking glucose relied heavily on costly or complex chemicals. Switching to simpler reactions helped bring those prices down and made self-testing more widespread. 2,3-Diaminophenazine stepped up as a handy color marker in the process, helping doctors and patients monitor blood sugar in real time. What’s at stake isn’t just convenience; reliable and affordable testing helps people fend off larger health worries before they grow.
Outside diabetes, researchers working on disease testing kits — think malaria, tuberculosis, cancer — often reach for 2,3-Diaminophenazine. It acts like a signal flare in enzyme-based tests, giving a strong, clear color change where disease signals turn up. That change isn’t easy magic; it needs a chemical like 2,3-Diaminophenazine to turn a silent reaction into something you can actually see, read, and measure. In developing regions, these kinds of tests cut through roadblocks that prevent early detection, keeping costs down without losing accuracy.
Most of the time, the end user doesn’t see the compound itself. It gets tucked away inside kits or solutions, letting someone prick a finger, dip a strip, and spot an answer with their own eyes. Low-income clinics and at-home test makers often prefer tools built from reliable, shelf-stable ingredients. 2,3-Diaminophenazine fits in without special storage needs, making it a steady choice in rugged conditions.
Every compound brings challenges. Lab workers follow safety routines, since repeated exposure can trigger skin or respiratory problems. Schools and small labs sometimes lean too hard on “all-purpose” chemicals without training, risking mishandling. Sharing clear instructions and basic protective gear can head off most trouble. Manufacturers ought to keep clear information handy, not just bury it in jargon.
As new diseases crop up and testing evolves, there’s pressure to improve every link in the chain, from raw ingredients to packaged test. Open data on how these chemicals react is a plus — more eyes checking results means safer, more accurate tools down the road. People in science should also look ahead to environmental effects, testing disposal methods instead of tossing everything into normal trash. Teamwork between researchers, makers, and clinics drives real progress.
A compound like 2,3-Diaminophenazine might not make headlines, but the way it makes invisible problems visible gives it weight beyond its chemistry. Reliable testing has changed daily life for millions; keeping these building blocks safe, affordable, and easy to use carries every bit as much value as splashier breakthroughs.
My first run-in with 2,3-Diaminophenazine came during grad school on a summer afternoon when the building’s ancient AC sputtered out. A bottle of deep yellow powder sat under a hood, sweating just like the students. That day, I learned the hard way that careless storage can turn a useful reagent into a ticking hazard.
Chemicals like 2,3-Diaminophenazine serve a purpose in research and industry, from dye synthesis to medical testing. Trouble brews when the environment encourages them to break down, vaporize, or react. Poor storage means safety issues, wasted money, and botched experiments. I’ve watched a day’s hard work dissolve because someone left a tightly capped bottle open in a humid corner. It’s a headache you don’t forget.
Dry, cool, and dark—those words became a mantra early in my time at the university. 2,3-Diaminophenazine doesn’t come with a lot of bells and whistles, but its stability takes a hit from heat, light, and moisture. Heat pushes up reactivity, light can spark photodegradation, and a little humidity is enough to clump the powder or start unwanted changes. I’ve seen crystalline chemicals melt together in a sticky mess just from humid storage, wasting pricey specialty reagents.
Sealed glass containers work best. In my corner of the lab, amber bottles line the shelves, each labeled twice. If you’ve ever mistaken one yellow powder for another, you don’t forget the value of clear labeling. Teflon-lined caps block air and water a lot better than cheap plastic. I toss in a small packet of desiccant, too. Silica gel does the trick for soaking up stray moisture, keeping powders dry for months.
Benchtop storage sets up problems. Lab benches catch sunlight and heating vents. That’s why chemical supply closets, far from windows, serve as the perfect home for 2,3-Diaminophenazine. My own lab stuck the flammables fridge just above freezing, with a dedicated shelf for reagents like this one. Cold slows chemical change. Most datasheets recommend between 2–8°C—not so cold that condensation forms inside bottles, not so warm that volatile compounds escape. Household fridges play host to things far less delicate, but the theory holds.
In research, a lost reagent means scrambling, expense reports, and risk of exposure. 2,3-Diaminophenazine can irritate eyes and skin. Once, a spill down the back of my glove led to an afternoon of burning discomfort. Simple goggles, gloves, and lab coats do more than keep supervisors happy—they protect you from unexpected splashes or puffs of powder. I make it a habit to check for leaks and damage every Friday, tossing anything with dodgy seals into the hazardous waste bin.
Investing in proper cabinets, reliable fridges, and airtight bottles may pinch budgets, but the alternative—chemical waste and health scares—runs much steeper. In group meetings, I’ve argued for automated temperature logs and humidity sensors. These gadgets send alerts if something drifts out of safe range, which saved our shelves once during a long weekend power outage. Some colleagues opt for vacuum-sealed packaging, especially if their climate runs toward the damp.
Even small labs can set minimum standards for chemical storage. Posting signs, training new students, and running occasional audits spark good habits. These steps won’t win awards, but they mean fewer ruined experiments and safer workspaces. In the end, a little care with 2,3-Diaminophenazine—just as with any specialty chemical—keeps researchers safe and science on track.
Anyone who’s ever peered inside a chemical storeroom knows the simple act of reading a label can spark questions that matter. Take 2,3-Diaminophenazine. You pick it up expecting a deep yellow powder, used by chemists in research and industry. But purity—what does that actually mean in real terms? On paper, numbers like 95%, 98%, or even a “reagent grade” sound clear, but they don’t always tell the whole story about how this compound behaves in a test tube or in a real-life process.
As someone who has run small-scale syntheses, purity often draws a line between a straightforward reaction and one that sends you scurrying to interpret surprising signals in your spectra. For 2,3-Diaminophenazine, even minor impurities like leftover precursors or environmental contaminants—things like aromatic amines or excessive water content—can set off unexpected by-products, throw off yield, or even grind the reaction to a halt. Some suppliers advertise over 98% purity for their analytical grade; sometimes, that's mostly true. Actual numbers tend to hover in the 97–99% range for lab use, with pharmaceutical or electronics suppliers occasionally promising even higher.
So, why does that matter? In organic chemistry labs and pharmaceutical research, even a couple points shy of absolute purity can pollute downstream synthesis. By-products can mimic real signal in thin-layer chromatography or slip past initial characterization. I once watched a colleague lose weeks on a single molecule, eventually tracing the trouble back to a bottle of “high purity” starting material that left a stubborn, invisible shadow in NMR and IR spectra.
Manufacturers typically measure purity using techniques like high-performance liquid chromatography (HPLC), gas chromatography (GC), and melting point analysis. These can sniff out common contaminants—sometimes as low as parts per million. Spectroscopy can flag fluorescence, a common trait of phenazines, to catch residues from incomplete synthesis. But number-chasing has a quirk: one supplier’s “99%” doesn’t always align with another’s testing standard. Some only subtract water or volatile solvents, leaving trace metals or inorganic salts out of the equation.
In academic settings, few researchers stop at a label. They’ll run their own melting point checks, infrared scans, or even elemental analysis to see whether there's an uninvited guest in the flask. This habit matters, especially if you’re trying to publish or build on previous work. Process chemists in industry will often batch test samples to avoid delays, since one contaminated batch can waste raw materials and time.
Consistency in purity isn’t just a checkbox for buyers—it impacts safety and reliability in medicine, battery research, and dye production. Quality control needs more than certificates; it requires transparent reporting, clear lot numbers, and technical support willing to discuss real results, not just numbers on a datasheet. Laboratories benefit from honest certificates of analysis and regular batch verifications. Suppliers earn trust more by sharing their full testing data, not only headline numbers.
For researchers, it pays to treat each new bottle as a variable, not a constant. Small pilot reactions and side-by-side quality checks can catch problems early. When possible, choosing suppliers with long-standing reputations and robust quality systems decreases headaches later on. My own experience says it’s better to over-check and avoid waste, especially when the outcome of an experiment—or a patient’s treatment—depends on purity that stays higher than just a marketing promise.
Some chemicals you bump into only in textbooks. Others might pop up in a university lab, or maybe in a factory making dyes or medical testing agents. 2,3-Diaminophenazine belongs to the second camp. With its deep yellow color, this compound has plenty of uses in labs, especially where scientists measure tiny amounts of things like hydrogen peroxide or iron. Some older dye recipes still list it as a possible ingredient, though these days, most folks outside of science circles have never heard its name.
Whether something is hazardous depends not just on what it can do, but on how people run into it. A knife in a drawer isn’t dangerous until someone pulls it out and misuses it. 2,3-Diaminophenazine comes with official warnings. This isn’t just paperwork or legal back-covering. It can irritate skin and eyes, and breathing the dust might bother your lungs. As with plenty of seemingly harmless powdery stuff, you don’t want to eat it, breathe it, or rub it in your eyes. That may sound like stating the obvious—yet plenty of lab techs discover the hard way that safety rules aren’t just for show.
Toxicology reports point out that 2,3-Diaminophenazine belongs in the same chemical family as several cancer-causing compounds. Scientists haven’t done long-term human studies for this chemical alone, but similar compounds used unwisely have landed people in the hospital. Some research suggests that amines like this one can trigger allergic reactions or worse if someone gets exposed day-in and day-out without decent ventilation or protective gear. Nobody needs that sort of surprise.
Most people don’t run into 2,3-Diaminophenazine at home or in the street. For the average person, the risk ranks just above worrying about tripping over lab coats. In research labs or manufacturing, though, chances of exposure aren’t just theoretical. I remember the first time I worked with a vivid yellow powder—my mentor didn’t just hand me gloves and goggles, she reminded me that a few minutes of carelessness could mean a day at the doctor’s office. She’d seen coworkers with red, itchy hands after skipping gloves or working in a rush. Over time, repeated exposure raises the stakes, sometimes in ways nobody expects.
The real problem crops up when corners get cut. Maybe the lab’s short on gloves, or the fume hood fan rattles instead of running strong. Even smart people make sloppy choices when deadlines loom. It’s not about alarmism—it’s about saving skin and lungs. Regulators and workplace safety folks need to keep pressing for training and regular safety checks. Labels on bottles should spell out real risks and practical steps, not just confusing numbers. If a chemical like this gets replaced with something less risky, all the better.
A single compound often shines a light on bigger patterns. 2,3-Diaminophenazine might not flood headlines, but its hazards aren’t unique. Taking shortcuts, rolling the dice on unknown chemicals, or ignoring best practices never pays off. Workplaces that treat harmful chemicals with the same seriousness they would open flames or high voltages see far fewer injuries. People don’t remember the times they dodged a bullet, but everyone remembers a bad burn—or worse.
Chemistry doesn't always shout at you; sometimes, it just throws down a stubborn challenge. 2,3-Diaminophenazine is quaint, but if you’ve ever worked with it, you know it can drive you up the wall. Anyone in a chemistry lab, student or seasoned chemist, has spent time eyeing a solid that sits like a brick in the bottom of a vial, refusing to dissolve. I’ve found myself in that spot often, scavenging for the right solvent, muttering at the bench.
2,3-Diaminophenazine stands out because of its two amino groups and that aromatic, fused-ring structure. Those amino groups look friendly, but they don’t always play nice with many common solvents. The structure makes for strong intermolecular forces—you feel the pain the moment you try to get it moving in solution.
You might grab a bottle of water out of habit. No luck—2,3-Diaminophenazine remains mostly insoluble in water, even if you heat things up or stir a storm. Ethanol and methanol? Results improve slightly, especially with methanol, but you won't see that vivid yellow-orange color unless you shake vigorously or use plenty of heat. Ethanol might soften things up, but most batches stay stubborn.
Now, reach for DMSO (dimethyl sulfoxide) or DMF (dimethylformamide) and the story changes. An old professor once told me, “If you don’t know what to do, throw DMSO at the problem.” He had a point—these polar aprotic solvents can do magic. 2,3-Diaminophenazine dissolves fairly well in DMSO, almost grudgingly obedient. DMF gets similar respect. The transparency of the solution still feels like a small victory every time.
Chloroform and dichloromethane do not usually help much. Ether, diethyl or petroleum-based, often gets ignored, and for good reason—the stuff just stays solid. Acetone works, but not as well as DMSO or DMF. It’s a game of patience and sometimes a bit of luck.
It’s tempting to reach for acids or bases—hydrochloric acid, sulfuric, or a bit of sodium hydroxide—but results vary. The molecule’s stability drops under harsh conditions, and you’re left cleaning up a worse mess than you started with. If you care about purity, you avoid this shortcut.
Learning to dissolve 2,3-Diaminophenazine gave me a new appreciation for “tried and true.” Someone once left a notebook on the bench detailing their mishaps—entries like "tried to dissolve in THF, nothing happened" or "heated in ethanol, still cloudy." That notebook meant more than any printed protocol. Most working chemists, if pressed, will echo the same advice: stick with DMSO or DMF, try mild heating, and don’t force it.
What makes this whole business important lies beyond the bench. Many modern industries, from dye manufacturing to analytical chemistry, depend on efficient, friendly solvents. Health, cost, and environmental headaches come right behind. DMSO works, but too much reliance on exotic solvents can bring storage headaches and waste concerns. Greener alternatives remain a goal, but for tough customers like 2,3-Diaminophenazine, we have more dreaming to do.
A safe and simple approach: keep DMSO and DMF nearby, and heat gently. Don't throw everything in the fume hood; respect what the molecule wants. Ask colleagues what has worked for them—a five-minute chat can save hours. Push for solvent recycling, limit waste, and look for greener blends when possible. The perfect solvent hasn’t shown up yet, but the small victories in the lab, whether it's a clear solution or shared notebook wisdom, still make chemistry worth every headache.
| Names | |
| Preferred IUPAC name | 5,10-Diaminophenazine |
| Pronunciation | /tuː θriː daɪˌæmɪnoʊfəˈnæziːn/ |
| Identifiers | |
| CAS Number | [92-54-6] |
| Beilstein Reference | 1662789 |
| ChEBI | CHEBI:53108 |
| ChEMBL | CHEMBL3217363 |
| ChemSpider | 170755 |
| DrugBank | DB04193 |
| ECHA InfoCard | ECHA InfoCard: 100.009.776 |
| EC Number | 1.10.3.10 |
| Gmelin Reference | 635595 |
| KEGG | C06223 |
| MeSH | D008926 |
| PubChem CID | 14753 |
| RTECS number | ST8650000 |
| UNII | W5K4N7A41J |
| UN number | UN3316 |
| CompTox Dashboard (EPA) | GHS11652 |
| Properties | |
| Chemical formula | C12H10N4 |
| Molar mass | molar mass of 2,3-Diaminophenazine is 210.23 g/mol |
| Appearance | Dark red to brown powder |
| Odor | Odorless |
| Density | 1.37 g/cm3 |
| Solubility in water | Insoluble in water |
| log P | 0.52 |
| Acidity (pKa) | 3.86 |
| Basicity (pKb) | 2.42 |
| Magnetic susceptibility (χ) | -65.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.776 |
| Viscosity | 1.34 cP (25°C) |
| Dipole moment | 1.52 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 186.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | 56.69 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4162 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed or in contact with skin. Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | C1=CC2=NC(=NC=C2C=C1)N |
| Signal word | Warning |
| Hazard statements | H302 + H315 + H319 + H335 |
| Precautionary statements | P280-P261-P305+P351+P338-P302+P352 |
| Flash point | 160 °C |
| Lethal dose or concentration | LD50 (oral, rat): >1000 mg/kg |
| LD50 (median dose) | LD50 (median dose): >500 mg/kg (rat, oral) |
| NIOSH | Not Established |
| PEL (Permissible) | Not established |
| REL (Recommended) | 100-500 mg/L |
| IDLH (Immediate danger) | IDLH for 2,3-Diaminophenazine: Not established |
| Related compounds | |
| Related compounds |
Neutral red Safranin Safranine O |